At 25°C the molar volume of water V is given by: V = 18.066 – 7.15 X 10ª P + 4.6 X 108 P², where V is in cm?/mol and P is in atm for Pressure between 0 and 1000 atm and (av Ст3 = 0.0045 + 1.4 x 10-6P - .K) mol Determine the work necessary at 25 °C to compress 1 mol of water from 1 atm to 1000 atm and the change in its internal energy.
At 25°C the molar volume of water V is given by: V = 18.066 – 7.15 X 10ª P + 4.6 X 108 P², where V is in cm?/mol and P is in atm for Pressure between 0 and 1000 atm and (av Ст3 = 0.0045 + 1.4 x 10-6P - .K) mol Determine the work necessary at 25 °C to compress 1 mol of water from 1 atm to 1000 atm and the change in its internal energy.
Related questions
Question
Please respond asap.
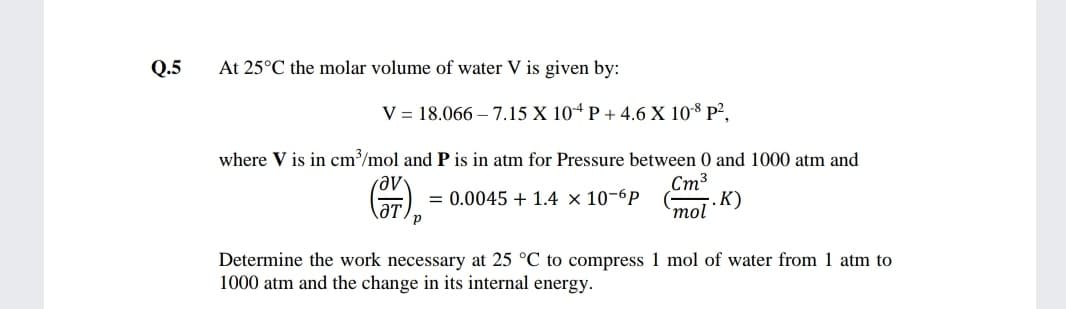
Transcribed Image Text:Q.5
At 25°C the molar volume of water V is given by:
V = 18.066 – 7.15 X 10ª P + 4.6 X 108 P²,
where V is in cm/mol and P is in atm for Pressure between 0 and 1000 atm and
Ст3
. К)
`mol
= 0.0045 + 1.4 × 10-6P
ƏT,
Determine the work necessary at 25 °C to compress 1 mol of water from 1 atm to
1000 atm and the change in its internal energy.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.