At 430°C, the equilibrium constant (Kp) for the reaction 2NO9) + 02(9) = 2NO2(9) Is 1.5 x 10°. In one experiment, the initial pressures of NO, O2, and NO2 are 2.1 x 103 atm, 1.1 x10? atm, and 0.14 atm, respectively. Calculate Q, and predict the direction that the net reaction will shift to reach equilibrium. (8 points) PE, Qp ра pb A,Bo
At 430°C, the equilibrium constant (Kp) for the reaction 2NO9) + 02(9) = 2NO2(9) Is 1.5 x 10°. In one experiment, the initial pressures of NO, O2, and NO2 are 2.1 x 103 atm, 1.1 x10? atm, and 0.14 atm, respectively. Calculate Q, and predict the direction that the net reaction will shift to reach equilibrium. (8 points) PE, Qp ра pb A,Bo
Chemistry: The Molecular Science
5th Edition
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:John W. Moore, Conrad L. Stanitski
Chapter12: Chemical Equilibrium
Section: Chapter Questions
Problem 60QRT
Related questions
Question
100%
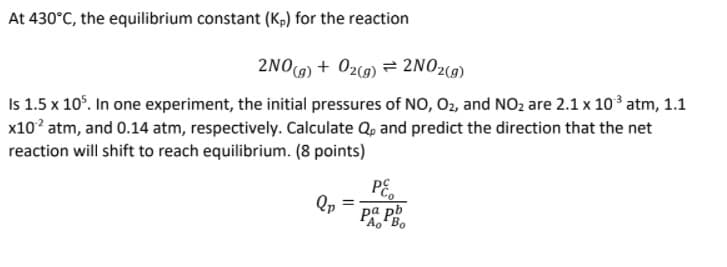
Transcribed Image Text:At 430°C, the equilibrium constant (Kp) for the reaction
2NO9) + 02(9) = 2NO2(9)
Is 1.5 x 10°. In one experiment, the initial pressures of NO, 02, and NO2 are 2.1 x 103 atm, 1.1
x10? atm, and 0.14 atm, respectively. Calculate Q, and predict the direction that the net
reaction will shift to reach equilibrium. (8 points)
PE,
Qp
ра pb
![The equilibrium constant (K.) for the formation of nitrosyl chloride, an orange-yellow
compound, from nitric oxide and molecular chlorine:
2NO) + Cl20) = 2NOC[9)
Is 6.5 x 10* at 35°C. In a certain experiment, 2.0 x102 mole of NO, 8.3 x103 mol of Cl2, and 6.8
moles of NOCI are mixed in a 2.0L flask. In which direction will the system proceed to reach
equilibrium? ([Concentration] = moles/volume) (8 points)](/v2/_next/image?url=https%3A%2F%2Fcontent.bartleby.com%2Fqna-images%2Fquestion%2Fe46f4908-7e86-4492-98b4-f701a1ee6e9f%2F11e5447e-1c4f-4bc9-b264-eb2883f94cfe%2Fzni1uaq_processed.jpeg&w=3840&q=75)
Transcribed Image Text:The equilibrium constant (K.) for the formation of nitrosyl chloride, an orange-yellow
compound, from nitric oxide and molecular chlorine:
2NO) + Cl20) = 2NOC[9)
Is 6.5 x 10* at 35°C. In a certain experiment, 2.0 x102 mole of NO, 8.3 x103 mol of Cl2, and 6.8
moles of NOCI are mixed in a 2.0L flask. In which direction will the system proceed to reach
equilibrium? ([Concentration] = moles/volume) (8 points)
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 3 images

Recommended textbooks for you

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning