At some point, the repulsive force grows so strong that the atoms stop approach and begin to move apart, causing the potential energy of the system to decrease. In the box below, explain this behavior: why do the atoms repel and move apart? (Hint: think about the electrons.)
At some point, the repulsive force grows so strong that the atoms stop approach and begin to move apart, causing the potential energy of the system to decrease. In the box below, explain this behavior: why do the atoms repel and move apart? (Hint: think about the electrons.)
Organic Chemistry: A Guided Inquiry
2nd Edition
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Andrei Straumanis
Chapter4: Polar Bonds, Polar Reactions
Section: Chapter Questions
Problem 10CTQ
Related questions
Question

Transcribed Image Text:At some point, the repulsive force grows so strong that
the atoms stop approach and begin to move apart,
causing the potential energy of the system to
decrease.
In the box below, explain this behavior: why do the
atoms repel and move apart? (Hint: think about the
electrons.)
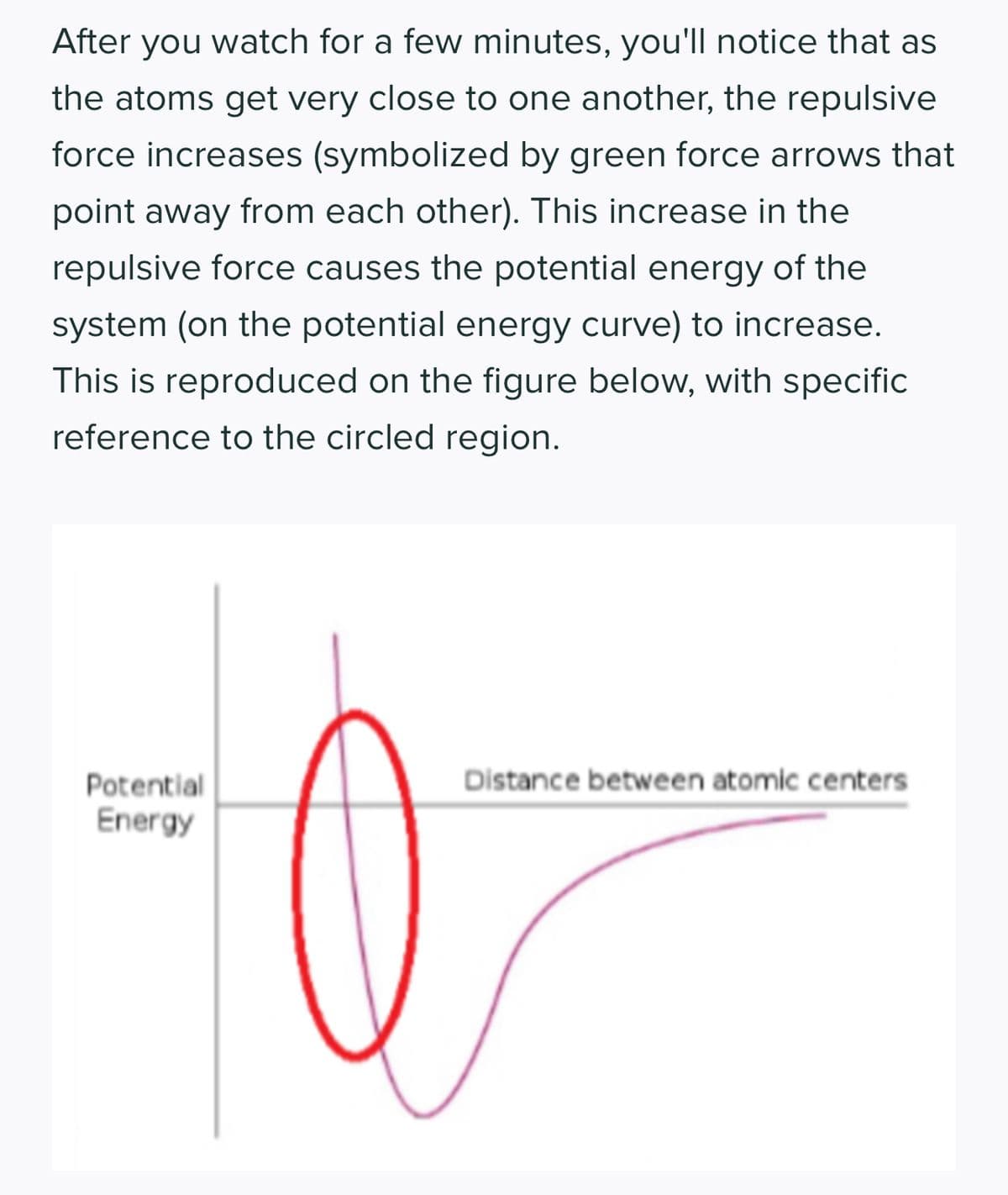
Transcribed Image Text:After you watch for a few minutes, you'll notice that as
the atoms get very close to one another, the repulsive
force increases (symbolized by green force arrows that
point away from each other). This increase in the
repulsive force causes the potential energy of the
system (on the potential energy curve) to increase.
This is reproduced on the figure below, with specific
reference to the circled region.
Potential
Energy
Distance between atomic centers
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning


Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning


Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning