At the beginning of an experiment, a sclentist has 164 grams of radioactive goo. After 255 minutes, her sample has decayed to 5.125 grams. What is the half-life of the goo in minutes? Find a formula for G(t), the amount of goo remaining at time t. G(t) = How many grams of goo will remain after 5 minutes? You may enter the exact value or round to 2 decimal places.
At the beginning of an experiment, a sclentist has 164 grams of radioactive goo. After 255 minutes, her sample has decayed to 5.125 grams. What is the half-life of the goo in minutes? Find a formula for G(t), the amount of goo remaining at time t. G(t) = How many grams of goo will remain after 5 minutes? You may enter the exact value or round to 2 decimal places.
Chemistry & Chemical Reactivity
10th Edition
ISBN:9781337399074
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Chapter25: Nuclear Chemistry
Section25.4: Rates Of Nuclear Decay
Problem 25.5CYU
Related questions
Question
100%
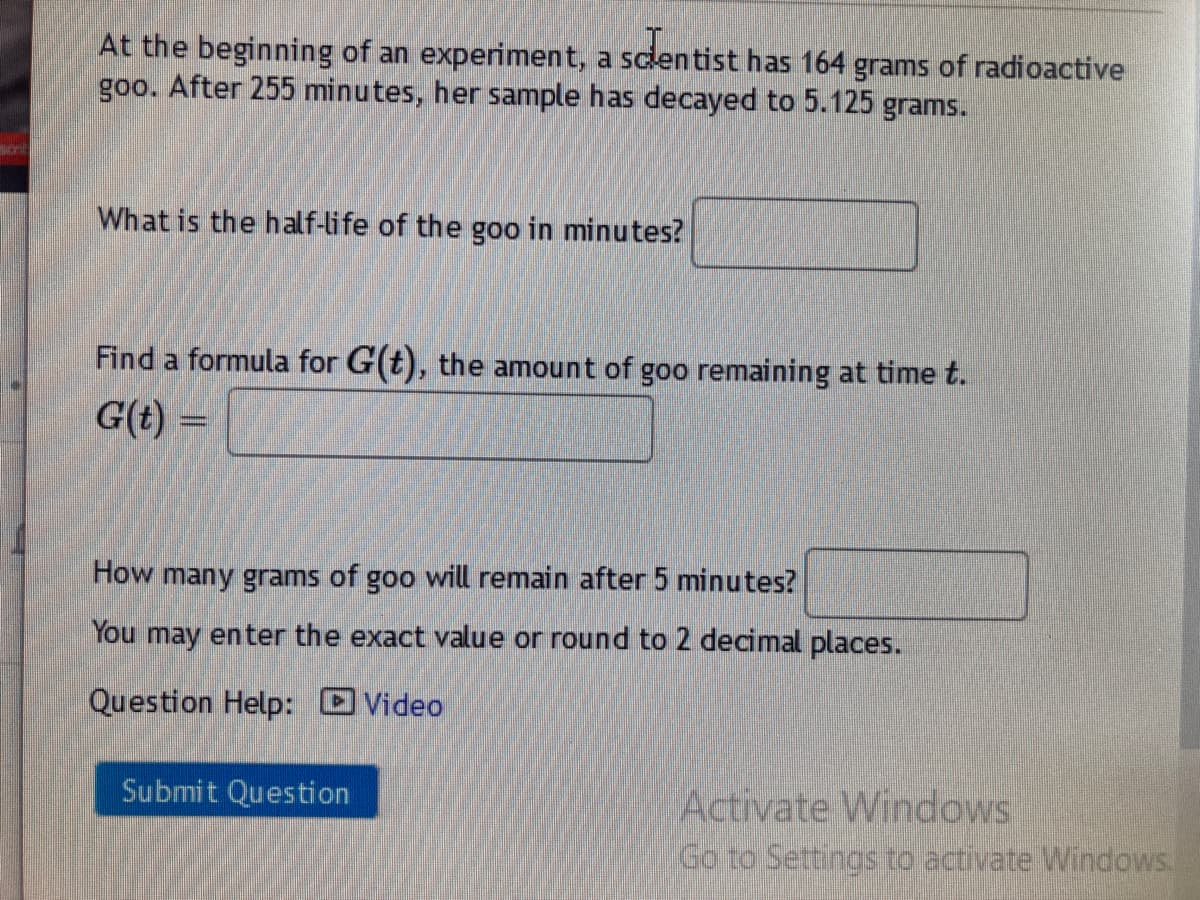
Transcribed Image Text:At the beginning of an experiment, a sclentist has 164 grams of radioactive
goo. After 255 minutes, her sample has decayed to 5.125 grams.
What is the half-life of the goo in minutes?
Find a formula for G(t), the amount of goo remaining at time t.
G(t) =
How many grams of goo will remain after 5 minutes?
You may enter the exact value or round to 2 decimal places.
Question Help: Video
Submit Question
Activate Windows
Go to Settings to activate Windows.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 4 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781285199023
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning