At what pH does EDTA become a strong complexing agent? * 04 06 8 10 Which best describes the characteristic of an indicator in a direct EDTA titration? Binds with the ligand and changes color when freed Binds with the metal and changes color when freed Changes color when binding with the complex formed The ligand itself is the indicator Which of the following does NOT form a 1:1 ratio with an EDTA molecule? * Li Mg Bi Al
At what pH does EDTA become a strong complexing agent? * 04 06 8 10 Which best describes the characteristic of an indicator in a direct EDTA titration? Binds with the ligand and changes color when freed Binds with the metal and changes color when freed Changes color when binding with the complex formed The ligand itself is the indicator Which of the following does NOT form a 1:1 ratio with an EDTA molecule? * Li Mg Bi Al
Chapter9: Complexometric And Precipitation Titrations
Section: Chapter Questions
Problem 1P
Related questions
Question
100%
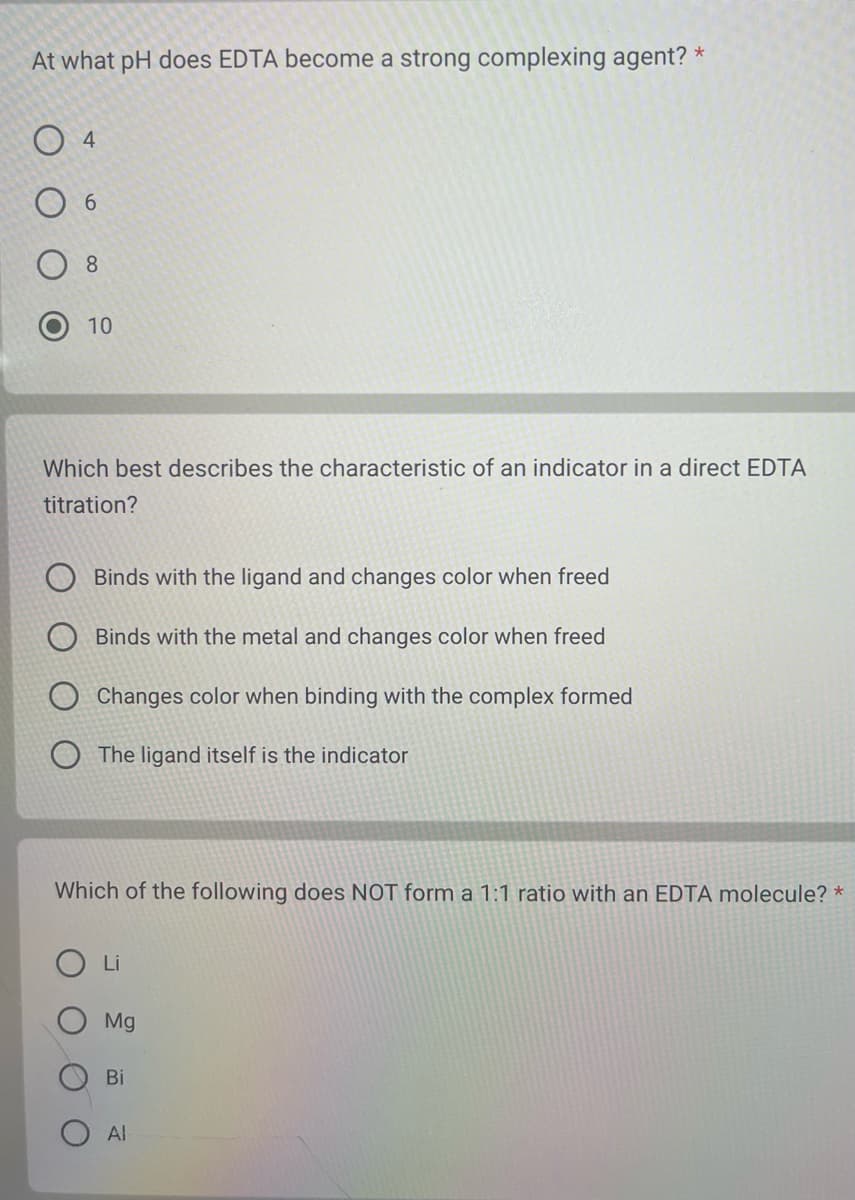
Transcribed Image Text:At what pH does EDTA become a strong complexing agent? *
04
6
8
10
Which best describes the characteristic of an indicator in a direct EDTA
titration?
Binds with the ligand and changes color when freed
Binds with the metal and changes color when freed
Changes color when binding with the complex formed
The ligand itself is the indicator
Which of the following does NOT form a 1:1 ratio with an EDTA molecule? *
Li
Mg
Bi
Al
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you



