Atomizer Bright light Oil droplet Figure 30.9 The Millikan oil drop experiment produced the first accurate direct measurement of the charge on electrons, one of the most fundamental constants in nature. Fine drops of oil become charged when sprayed. Their movement is observed between metal plates with a potential applied to oppose the gravitational force. The balance of gravitational and electric forces allows the calculation of the charge on a drop. The charge is found to be quantized in units of –1.6 x 10-19 C, thus determining directly the charge of the excess and missing electrons on the oil drops.
Atomizer Bright light Oil droplet Figure 30.9 The Millikan oil drop experiment produced the first accurate direct measurement of the charge on electrons, one of the most fundamental constants in nature. Fine drops of oil become charged when sprayed. Their movement is observed between metal plates with a potential applied to oppose the gravitational force. The balance of gravitational and electric forces allows the calculation of the charge on a drop. The charge is found to be quantized in units of –1.6 x 10-19 C, thus determining directly the charge of the excess and missing electrons on the oil drops.
College Physics
1st Edition
ISBN:9781938168000
Author:Paul Peter Urone, Roger Hinrichs
Publisher:Paul Peter Urone, Roger Hinrichs
Chapter30: Atomic Physics
Section: Chapter Questions
Problem 56PE: Integrated Concepts In a Millikan oil-drop experiment using a setup like that in Figure 30.9, a...
Related questions
Concept explainers
Question
Integrated Concepts
In a Millikan oil-drop experiment using a setup like that in Figure 30.9, a 500-V potential difference is applied to plates separated by 2.50 cm.What is the mass of an oil drop having two extra electrons that is suspended motionless by the field between the plates?
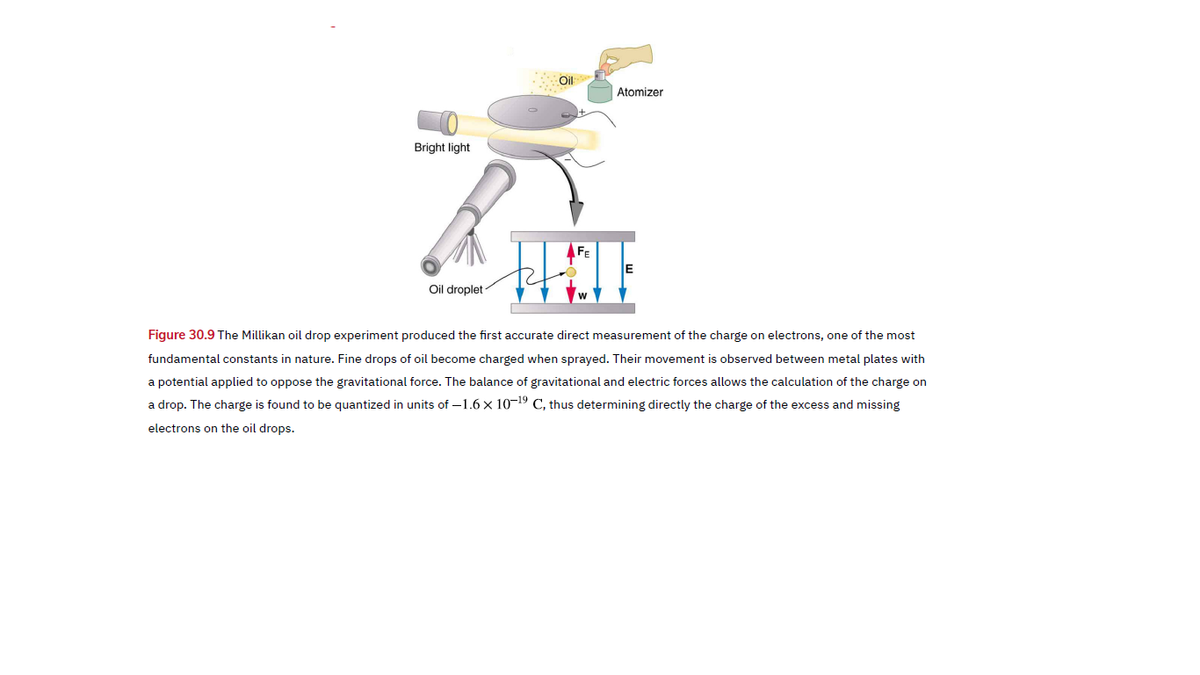
Transcribed Image Text:Atomizer
Bright light
Oil droplet
Figure 30.9 The Millikan oil drop experiment produced the first accurate direct measurement of the charge on electrons, one of the most
fundamental constants in nature. Fine drops of oil become charged when sprayed. Their movement is observed between metal plates with
a potential applied to oppose the gravitational force. The balance of gravitational and electric forces allows the calculation of the charge on
a drop. The charge is found to be quantized in units of –1.6 x 10-19 C, thus determining directly the charge of the excess and missing
electrons on the oil drops.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, physics and related others by exploring similar questions and additional content below.Recommended textbooks for you

College Physics
Physics
ISBN:
9781938168000
Author:
Paul Peter Urone, Roger Hinrichs
Publisher:
OpenStax College

College Physics
Physics
ISBN:
9781305952300
Author:
Raymond A. Serway, Chris Vuille
Publisher:
Cengage Learning

University Physics Volume 3
Physics
ISBN:
9781938168185
Author:
William Moebs, Jeff Sanny
Publisher:
OpenStax

College Physics
Physics
ISBN:
9781938168000
Author:
Paul Peter Urone, Roger Hinrichs
Publisher:
OpenStax College

College Physics
Physics
ISBN:
9781305952300
Author:
Raymond A. Serway, Chris Vuille
Publisher:
Cengage Learning

University Physics Volume 3
Physics
ISBN:
9781938168185
Author:
William Moebs, Jeff Sanny
Publisher:
OpenStax

Glencoe Physics: Principles and Problems, Student…
Physics
ISBN:
9780078807213
Author:
Paul W. Zitzewitz
Publisher:
Glencoe/McGraw-Hill