Attempts Force Completion This test can be saved and resumed later. Your answers are saved automatically. * Question Completion Status: L A Moving to another question will save this response. Question 3 For the reaction at 20. °C, NH3(aq) + + H+ (aq) t NH4(aq) + The equilibrium constant is calculated to be K = 4.5 x 108 Question 3 of 6 >>> 1 points Save Answer If a base (NaOH) was added to the mixture, the hydroxide ion, OH, would react with the H+ to form water. This would decrease the concentration of H+ in the solution. In which direction would the equilibrium shift, to the left, to the right, or neither? a. Equilibrium would shift to the right. Ob. Equilibrium would shift to the left. Oc. Equilibrium would not shift. L A Moving to another question will save this response. Question 3 of 6 >>> A
Attempts Force Completion This test can be saved and resumed later. Your answers are saved automatically. * Question Completion Status: L A Moving to another question will save this response. Question 3 For the reaction at 20. °C, NH3(aq) + + H+ (aq) t NH4(aq) + The equilibrium constant is calculated to be K = 4.5 x 108 Question 3 of 6 >>> 1 points Save Answer If a base (NaOH) was added to the mixture, the hydroxide ion, OH, would react with the H+ to form water. This would decrease the concentration of H+ in the solution. In which direction would the equilibrium shift, to the left, to the right, or neither? a. Equilibrium would shift to the right. Ob. Equilibrium would shift to the left. Oc. Equilibrium would not shift. L A Moving to another question will save this response. Question 3 of 6 >>> A
Chemistry by OpenStax (2015-05-04)
1st Edition
ISBN:9781938168390
Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Chapter15: Equilibria Of Other Reaction Classes
Section: Chapter Questions
Problem 109E: The following question is taken from a Chemistry Advanced Placement Examination and is used with the...
Related questions
Question
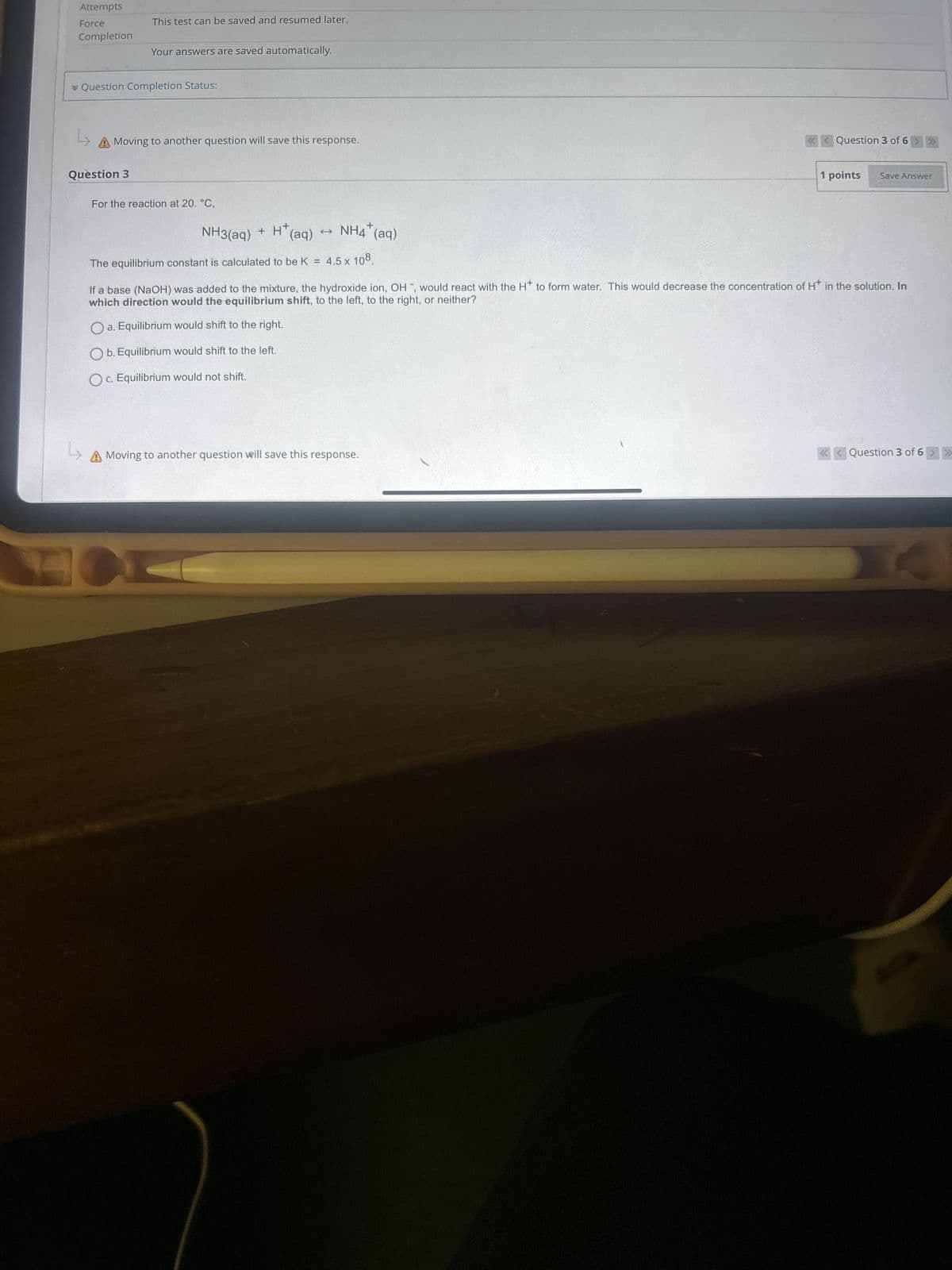
Transcribed Image Text:Attempts
Force
Completion
This test can be saved and resumed later.
Your answers are saved automatically.
* Question Completion Status:
L
A Moving to another question will save this response.
Question 3
For the reaction at 20. °C,
NH3(aq) +
+ H+
(aq)
t
NH4(aq)
+
The equilibrium constant is calculated to be K =
4.5 x 108
Question 3 of 6 >>>
1 points
Save Answer
If a base (NaOH) was added to the mixture, the hydroxide ion, OH, would react with the H+ to form water. This would decrease the concentration of H+ in the solution. In
which direction would the equilibrium shift, to the left, to the right, or neither?
a. Equilibrium would shift to the right.
Ob. Equilibrium would shift to the left.
Oc. Equilibrium would not shift.
L
A Moving to another question will save this response.
Question 3 of 6 >>>
A
AI-Generated Solution
Unlock instant AI solutions
Tap the button
to generate a solution
Recommended textbooks for you

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning
