(b) Shown below are two schematic band diagrams for silicon. Answer the following questions: (i) For phosphorous-doped silicon, draw a dashed line denoting the location of the additional state(s) created by the phosphorus at room temperature. Explain your answer. (ii) For phosphorous-doped silicon, draw a dashed line denoting the location of electrons donated by the phosphorus at room temperature. Explain your answer. Conduction Conduction band band Band Band дар gap Valence Valence band band (i) (ii)
(b) Shown below are two schematic band diagrams for silicon. Answer the following questions: (i) For phosphorous-doped silicon, draw a dashed line denoting the location of the additional state(s) created by the phosphorus at room temperature. Explain your answer. (ii) For phosphorous-doped silicon, draw a dashed line denoting the location of electrons donated by the phosphorus at room temperature. Explain your answer. Conduction Conduction band band Band Band дар gap Valence Valence band band (i) (ii)
Principles of Modern Chemistry
8th Edition
ISBN:9781305079113
Author:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Chapter21: Structure And Bonding In Solids
Section: Chapter Questions
Problem 39P
Related questions
Question
100%
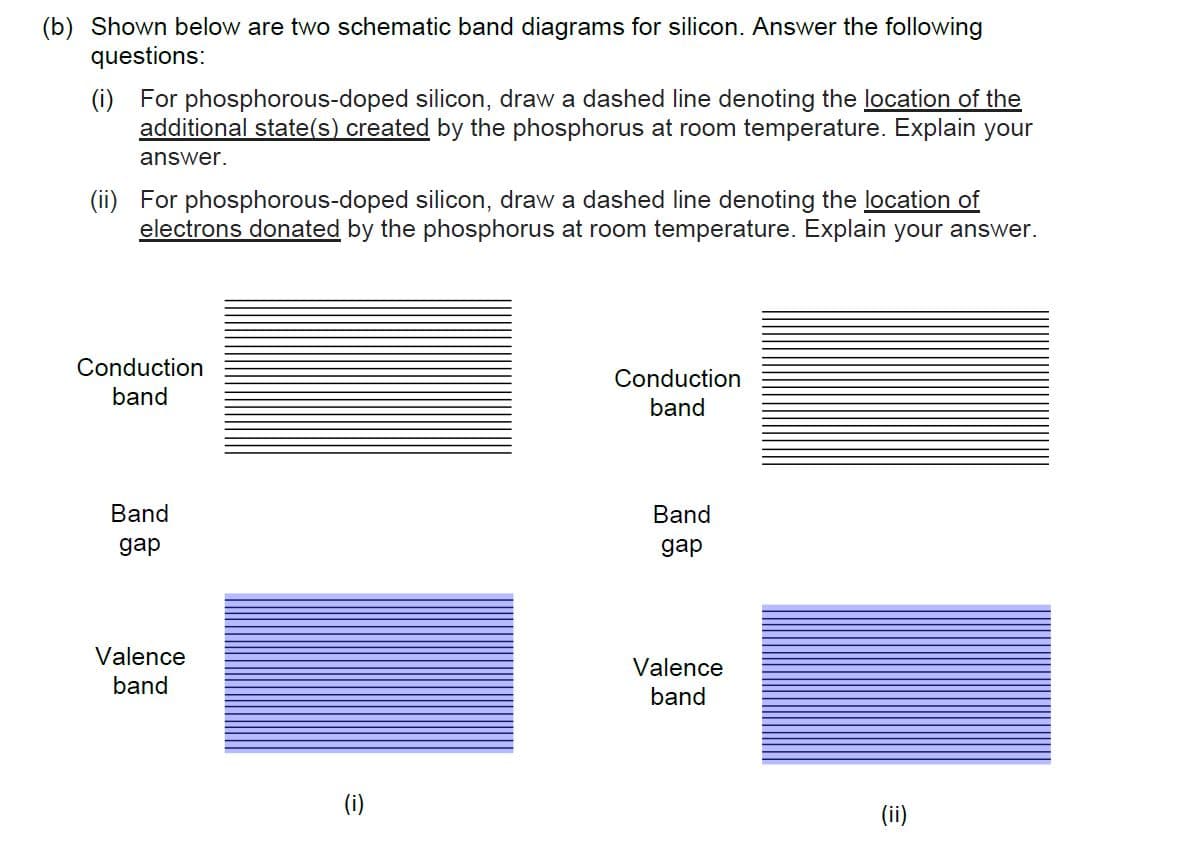
Transcribed Image Text:(b) Shown below are two schematic band diagrams for silicon. Answer the following
questions:
(i) For phosphorous-doped silicon, draw a dashed line denoting the location of the
additional state(s) created by the phosphorus at room temperature. Explain your
answer.
(ii) For phosphorous-doped silicon, draw a dashed line denoting the location of
electrons donated by the phosphorus at room temperature. Explain your answer.
Conduction
Conduction
band
band
Band
Band
дар
gap
Valence
Valence
band
band
(i)
(ii)
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 3 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning