Benzene (C6H6) is an organic compound, and KCl is an ionic compound. The sum of the masses of the atoms in each empirical formula is approximately the same. How would you expect the two to compare with regard to melting point, type of bonding, rate of evaporation, and structure? Fill in the blanks below by choosing the correct compound from the drop-down menu. a. The melting point of + will be much higher than that of b. + is a covalent organic compound; * is an ionic inorganic compound. c. The rate of evaporation of + will be many orders of magnitude greater than that of d. +: weak intermolecular interactions cause it to be a volatile liquid at room temperature.
Benzene (C6H6) is an organic compound, and KCl is an ionic compound. The sum of the masses of the atoms in each empirical formula is approximately the same. How would you expect the two to compare with regard to melting point, type of bonding, rate of evaporation, and structure? Fill in the blanks below by choosing the correct compound from the drop-down menu. a. The melting point of + will be much higher than that of b. + is a covalent organic compound; * is an ionic inorganic compound. c. The rate of evaporation of + will be many orders of magnitude greater than that of d. +: weak intermolecular interactions cause it to be a volatile liquid at room temperature.
Chemistry: An Atoms First Approach
2nd Edition
ISBN:9781305079243
Author:Steven S. Zumdahl, Susan A. Zumdahl
Publisher:Steven S. Zumdahl, Susan A. Zumdahl
Chapter3: Bonding: General Concepts
Section: Chapter Questions
Problem 20Q: Some of the important properties of ionic compounds are as follows: i. low electrical conductivity...
Related questions
Question
100%
No 12
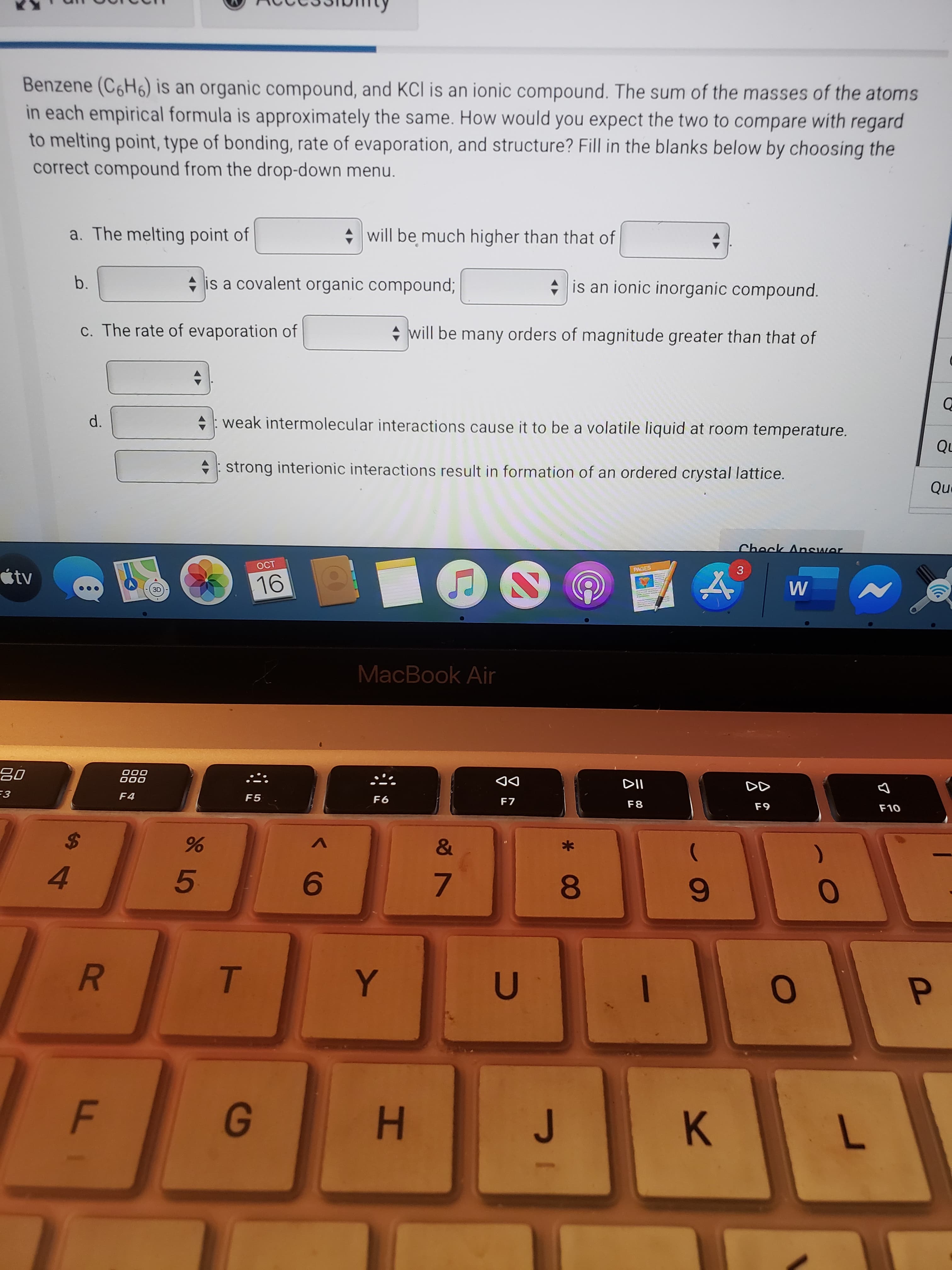
Transcribed Image Text:Benzene (C6H6) is an organic compound, and KCl is an ionic compound. The sum of the masses of the atoms
in each empirical formula is approximately the same. How would you expect the two to compare with regard
to melting point, type of bonding, rate of evaporation, and structure? Fill in the blanks below by choosing the
correct compound from the drop-down menu.
a. The melting point of
+ will be much higher than that of
b.
+ is a covalent organic compound;
* is an ionic inorganic compound.
c. The rate of evaporation of
+ will be many orders of magnitude greater than that of
d.
+: weak intermolecular interactions cause it to be a volatile liquid at room temperature.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

General, Organic, and Biological Chemistry
Chemistry
ISBN:
9781285853918
Author:
H. Stephen Stoker
Publisher:
Cengage Learning

Living By Chemistry: First Edition Textbook
Chemistry
ISBN:
9781559539418
Author:
Angelica Stacy
Publisher:
MAC HIGHER