Organic Chemistry: A Guided Inquiry
2nd Edition
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Andrei Straumanis
Chapter13: Substitution
Section: Chapter Questions
Problem 2E
Related questions
Question
Draw the products for the addition reactions below in the boxes. Then, draw the FULL electron-pushing mechanism for the reactions, including all intermediates (with formal charges and lone pairs of electrons) and all electron pushing arrows. Label the electrophile and nucleophile in each step.
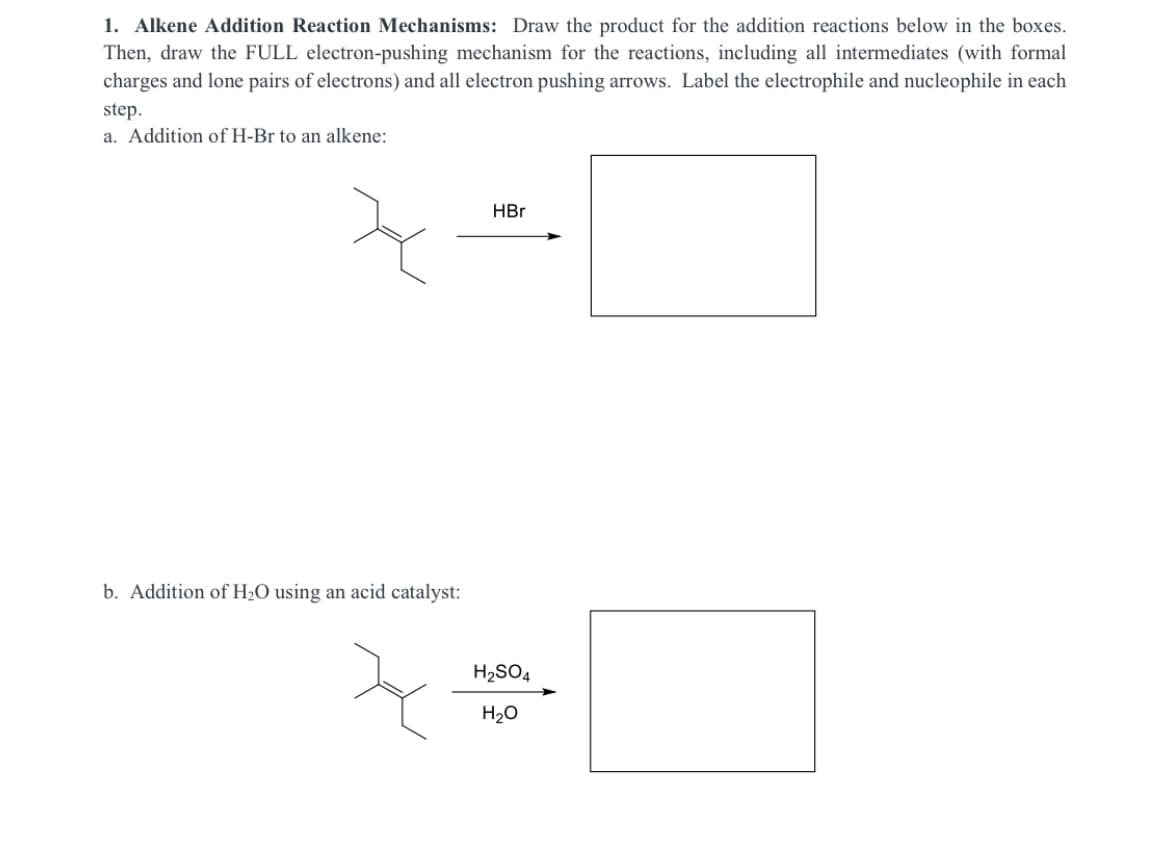
Transcribed Image Text:1. Alkene Addition Reaction Mechanisms: Draw the product for the addition reactions below in the boxes.
Then, draw the FULL electron-pushing mechanism for the reactions, including all intermediates (with formal
charges and lone pairs of electrons) and all electron pushing arrows. Label the electrophile and nucleophile in each
step.
a. Addition of H-Br to an alkene:
HBr
b. Addition of H2O using an acid catalyst:
H2SO4
H20
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Recommended textbooks for you

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning