B. Calculate the pH after 17.50 mL of NAOH is added to 35.00 mL of acetic acid using the concentration assigned in Beyond Labz: • First assume the acid-base neutralization reaction is irreversible and goes to completion: HC2H;O2 (ag) + OH° (ag) - H2O (1) + C;H;O;" (ag) Moles before reaction Change in moles Moles remaining after reaction • Then equilibrium is established HC;H;O2 (ag) 2H* (ag) + C;H;O2 (ag) Initial Concentration (M) Change in Concentration (M) Equilibrium Concentration (M) E Equilibrium Concentration, Calculated (M) Show your work here or replicate this on a separate piece of paper. Number and label each calculation, include units, and show the math performed to get your answer. Report answers with correct units and significant figures.
B. Calculate the pH after 17.50 mL of NAOH is added to 35.00 mL of acetic acid using the concentration assigned in Beyond Labz: • First assume the acid-base neutralization reaction is irreversible and goes to completion: HC2H;O2 (ag) + OH° (ag) - H2O (1) + C;H;O;" (ag) Moles before reaction Change in moles Moles remaining after reaction • Then equilibrium is established HC;H;O2 (ag) 2H* (ag) + C;H;O2 (ag) Initial Concentration (M) Change in Concentration (M) Equilibrium Concentration (M) E Equilibrium Concentration, Calculated (M) Show your work here or replicate this on a separate piece of paper. Number and label each calculation, include units, and show the math performed to get your answer. Report answers with correct units and significant figures.
Chemical Principles in the Laboratory
11th Edition
ISBN:9781305264434
Author:Emil Slowinski, Wayne C. Wolsey, Robert Rossi
Publisher:Emil Slowinski, Wayne C. Wolsey, Robert Rossi
Chapter21: Rates Of Chemical Reactions, Ii. A Clock Reaction
Section: Chapter Questions
Problem 2ASA
Related questions
Question
Acetic Acid = 0.1033 M
Ka = 1.8 x 10-5
Sodium hydroxide = 0.1104 M
The first picture are the data, if needed
second picture are the ones needs to be solved
![HC2H3O2
H+
+
C2H302-
Initial
0.1033
Change
Equilibrium
1. Initial concentration [HC2H3O2]o = 0.1033 M
-X
+X
+X
0.1033-x
X
[H*][C2H307]
2. Ka symbolic expression: Ka
[HC2H302]
x2
3. Ka numerical equation with x: 1.8 x 10-5
0.1033-x
4. Quadratic formula method
a. quadratic equation: x2 + 1.8x10-5x – 1.86x10-6 = 0
%3D
-1.8x10-5+V (1.8x10-5)2-4(1)(-1.86×10-6)
b. quadratic formula: x =
2(1)
x = 1.35x10-3 M; -1.37x103 M (only positive root is used)
5. Assumption (5% rule) method
a. equation for x: x =
V(1.8 x 10-5)(0.1033) = 1.36 × 10-3M
1.36x10-3 M
b. % ionization =
x 100 = 1.32%
0.1033 M
6. Equilibrium concentrations
a. [HC2H3O2leg = 0.1033 – 1.35x10-3 = 0.1020 M
%3D
%3D
b. [H*]eg = 1.35x10-3 M
Jeq
c. [C2H3O2]eg = 1.35x10-3 M
7. pH = -log[H*] = -log(1.35x10 3) = 2.87](/v2/_next/image?url=https%3A%2F%2Fcontent.bartleby.com%2Fqna-images%2Fquestion%2F378a66cd-977d-4b08-83be-b0cd331b8abe%2Fab3c6acf-f131-4fcc-9773-ccae6bc9137c%2F9e5zgdb_processed.jpeg&w=3840&q=75)
Transcribed Image Text:HC2H3O2
H+
+
C2H302-
Initial
0.1033
Change
Equilibrium
1. Initial concentration [HC2H3O2]o = 0.1033 M
-X
+X
+X
0.1033-x
X
[H*][C2H307]
2. Ka symbolic expression: Ka
[HC2H302]
x2
3. Ka numerical equation with x: 1.8 x 10-5
0.1033-x
4. Quadratic formula method
a. quadratic equation: x2 + 1.8x10-5x – 1.86x10-6 = 0
%3D
-1.8x10-5+V (1.8x10-5)2-4(1)(-1.86×10-6)
b. quadratic formula: x =
2(1)
x = 1.35x10-3 M; -1.37x103 M (only positive root is used)
5. Assumption (5% rule) method
a. equation for x: x =
V(1.8 x 10-5)(0.1033) = 1.36 × 10-3M
1.36x10-3 M
b. % ionization =
x 100 = 1.32%
0.1033 M
6. Equilibrium concentrations
a. [HC2H3O2leg = 0.1033 – 1.35x10-3 = 0.1020 M
%3D
%3D
b. [H*]eg = 1.35x10-3 M
Jeq
c. [C2H3O2]eg = 1.35x10-3 M
7. pH = -log[H*] = -log(1.35x10 3) = 2.87
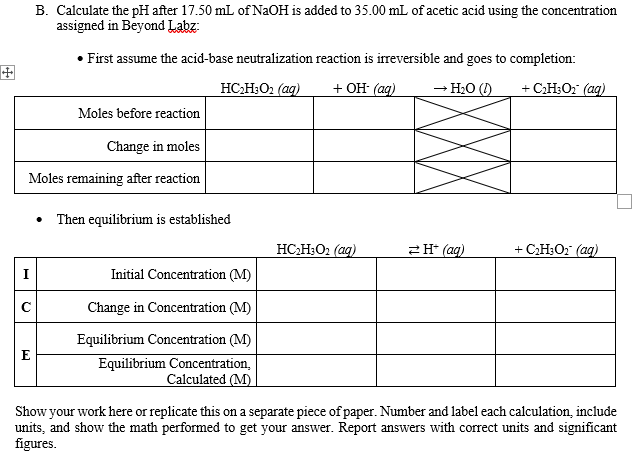
Transcribed Image Text:B. Calculate the pH after 17.50 mL of N2OH is added to 35.00 mL of acetic acid using the concentration
assigned in Beyond Labz:
• First assume the acid-base neutralization reaction is irreversible and goes to completion:
田
HC;H;O2 (ag)
+ Он (ag)
→ H2O ()
+ C;H3O2 (aq)
Moles before reaction
Change in moles
Moles remaining after reaction
Then equilibrium is established
HC;H;O2 (ag)
2H* (ag)
+ C;H;O2 (aq)
I
Initial Concentration (M)
Change in Concentration (M)
Equilibrium Concentration (M)
E
Equilibrium Concentration,
Calculated (M)
Show your work here or replicate this on a separate piece of paper. Number and label each calculation, include
units, and show the math performed to get your answer. Report answers with correct units and significant
figures.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 7 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemical Principles in the Laboratory
Chemistry
ISBN:
9781305264434
Author:
Emil Slowinski, Wayne C. Wolsey, Robert Rossi
Publisher:
Brooks Cole


Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Chemical Principles in the Laboratory
Chemistry
ISBN:
9781305264434
Author:
Emil Slowinski, Wayne C. Wolsey, Robert Rossi
Publisher:
Brooks Cole


Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning