B. Consider the same ideal gas within an adiabatically sealed container. Suppose that the ideal gas is initially in an equilibrium state with volume Va and temperature Tr. Suppose that the ideal gas then undergoes a free expansion (i.e., the gas expands against a vacuum) from Va → Vz.
B. Consider the same ideal gas within an adiabatically sealed container. Suppose that the ideal gas is initially in an equilibrium state with volume Va and temperature Tr. Suppose that the ideal gas then undergoes a free expansion (i.e., the gas expands against a vacuum) from Va → Vz.
Physical Chemistry
2nd Edition
ISBN:9781133958437
Author:Ball, David W. (david Warren), BAER, Tomas
Publisher:Ball, David W. (david Warren), BAER, Tomas
Chapter2: The First Law Of Thermodynamics
Section: Chapter Questions
Problem 2.65E: A sample of an ideal diatomic gas is compressed adiabatically and reversibly to double its initial...
Related questions
Question
if w=0, q= (H2-H1)-nR(T2-T1), and delta H=q:
Determine final volume and temperature of the gas.
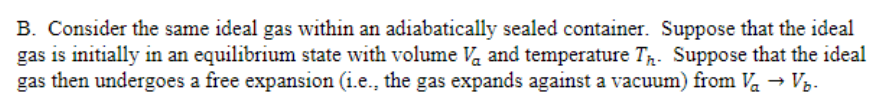
Transcribed Image Text:B. Consider the same ideal gas within an adiabatically sealed container. Suppose that the ideal
gas is initially in an equilibrium state with volume Va and temperature Tr. Suppose that the ideal
gas then undergoes a free expansion (i.e., the gas expands against a vacuum) from Va → Vz.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 3 steps

Recommended textbooks for you

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning