(b. iron(III) ion (c) oxide ion (d) iron metal For the reaction in problem 15, the substance being reduced is: (a) aluminum (d) iron metal 16. (b) iron(III) ion (c) oxide ion 17. The reaction in problem 15 can also be classified as a: (b) decomposition reaction (a) douh1e renlagoman (a) combination reaction (c) single replacement reaction
(b. iron(III) ion (c) oxide ion (d) iron metal For the reaction in problem 15, the substance being reduced is: (a) aluminum (d) iron metal 16. (b) iron(III) ion (c) oxide ion 17. The reaction in problem 15 can also be classified as a: (b) decomposition reaction (a) douh1e renlagoman (a) combination reaction (c) single replacement reaction
Principles of Modern Chemistry
8th Edition
ISBN:9781305079113
Author:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Chapter15: Acid–base Equilibria
Section: Chapter Questions
Problem 98AP
Related questions
Question
#18, explain
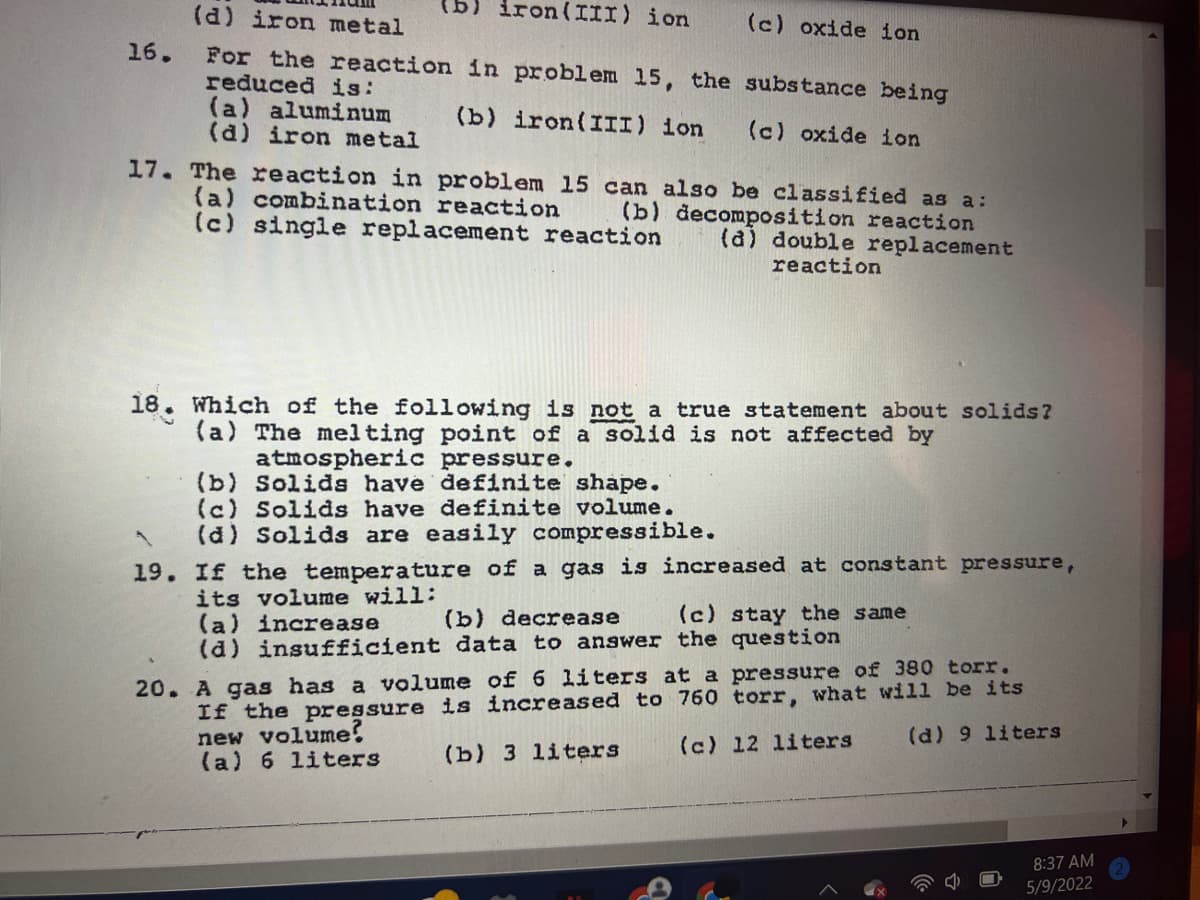
Transcribed Image Text:(d) iron metal
iron(III) ion
(c) oxide ion
g)
16.
For the reaction in problem 15, the substance being
reduced is:
(a) aluminum
(d) iron metal
(b) iron(III) ion
(c) oxide ion
17. The reaction in problem 15 can also be classified as a:
(a) combination reaction
(c) single replacement reaction
(b) decomposition reaction
(a) double replacement
reaction
18. Which of the following is not a true statement about solids?
(a) The melting point of a solid is not affected by
atmospheric pressure.
(b) Solids have definite shape.
(c) Solids have definite volume.
(d) Solids are easily compressible.
19. If the temperature of a gas is increased at constant pressure,
its volume will:
(a) increase
(d) insufficient data to answer the question
(b) decrease
(c) stay the same
If the pressure is increased to 760 torr, what will be its
new volume?
(a) 6 liters
20. A gas has a volume of 6 liters at a pressure of 380 torr.
(c) 12 liters
(d) 9 liters
(b) 3 liters
8:37 AM
5/9/2022
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning