B. Solubility of KNO3 1. Mass of 2. Mass of Container + KNO, 4. Mass of 3. Temperature 5. Solubility When Crystals (g KNO,/ Appear Container KNO, 100 mL H20) Tared 3.00g 38.0°C 4.00g 46.0°C 5.00g 55.0°C 6.00g 65.0°C 7.00g 71.0°C 6. Graph for the solubility of KNO, vs. temperature (°C) Q2 According to your graph, what is the effect of increasing temperature on the solubility of KNO,? Questions and Problems Q3 According to your graph, what is the solubility of KNO, at 65°C? Q4 According to your graph, at what temperature does KNO, have a solubility of 120 g/100 mL?
B. Solubility of KNO3 1. Mass of 2. Mass of Container + KNO, 4. Mass of 3. Temperature 5. Solubility When Crystals (g KNO,/ Appear Container KNO, 100 mL H20) Tared 3.00g 38.0°C 4.00g 46.0°C 5.00g 55.0°C 6.00g 65.0°C 7.00g 71.0°C 6. Graph for the solubility of KNO, vs. temperature (°C) Q2 According to your graph, what is the effect of increasing temperature on the solubility of KNO,? Questions and Problems Q3 According to your graph, what is the solubility of KNO, at 65°C? Q4 According to your graph, at what temperature does KNO, have a solubility of 120 g/100 mL?
Physical Chemistry
2nd Edition
ISBN:9781133958437
Author:Ball, David W. (david Warren), BAER, Tomas
Publisher:Ball, David W. (david Warren), BAER, Tomas
Chapter7: Equilibria In Multiple-component Systems
Section: Chapter Questions
Problem 7.38E
Related questions
Question
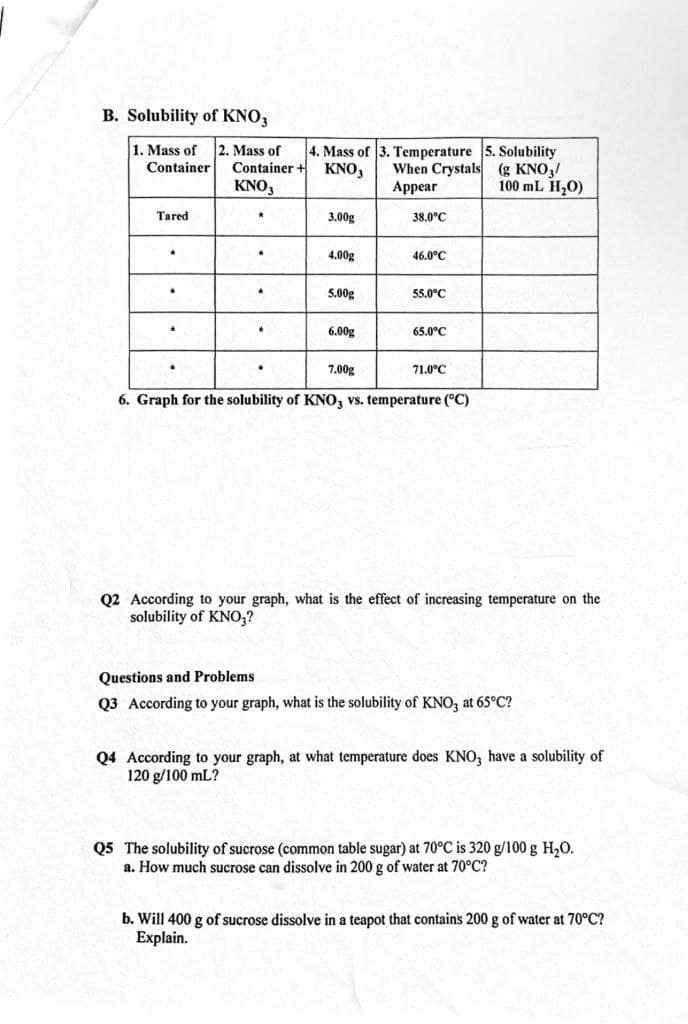
Transcribed Image Text:B. Solubility of KNO,
2. Mass of
Container +
KNO3
1. Mass of
4. Mass of
. Temperature 5. Solubility
When Crystals (g KNO,/
Appear
Container
KNO,
100 mL H20)
Tared
3.00g
38.0°C
4.00g
46.0°C
5.00g
55.0°C
6.00g
65.0°C
7.00g
71.0°C
6. Graph for the solubility of KNO, vs. temperature (°C)
Q2 According to your graph, what is the effect of increasing temperature on the
solubility of KNO,?
Questions and Problems
Q3 According to your graph, what is the solubility of KNO, at 65°C?
Q4 According to your graph, at what temperature does KNO, have a solubility of
120 g/100 mL?
Q5 The solubility of sucrose (common table sugar) at 70°C is 320 g/100 g H,0.
a. How much sucrose can dissolve in 200 g of water at 70°C?
b. Will 400 g of sucrose dissolve in a teapot that contains 200 g of water at 70°C?
Explain.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning