Barium carbonate is commercially important as a basis for the manufacture of other barium compounds. In its manufacture, barium sulfide is first prepared by heating the natural sulfate, barytes with carbon. The barium sulfide is extracted from this mass with water and the solution treated with sodium carbonate to precipitate the carbonate of barium. In the operation of such a process it is found that the solution of barium sulfide formed contains also some calcium sulfide, originating from impurities in the barytes. The solution is treated with sodium carbonate, and the precipitated mass of calcium and barium carbonates is filtered off. It is found that 16.45 kg of dry precipitate are removed from each 100 kg of filtrate collected. The analysis of the precipitate is: 9.9% CaCO; and 90.1% BaCO3. The analysis of the filtrate is 6.85% Na,S, 2.25% Na2CO; and 90.9% H2O. The sodium carbonate for precipitation was added in the form of anhydrous soda ash which contained CaCO3 as an impurity. Calculate:
Barium carbonate is commercially important as a basis for the manufacture of other barium compounds. In its manufacture, barium sulfide is first prepared by heating the natural sulfate, barytes with carbon. The barium sulfide is extracted from this mass with water and the solution treated with sodium carbonate to precipitate the carbonate of barium. In the operation of such a process it is found that the solution of barium sulfide formed contains also some calcium sulfide, originating from impurities in the barytes. The solution is treated with sodium carbonate, and the precipitated mass of calcium and barium carbonates is filtered off. It is found that 16.45 kg of dry precipitate are removed from each 100 kg of filtrate collected. The analysis of the precipitate is: 9.9% CaCO; and 90.1% BaCO3. The analysis of the filtrate is 6.85% Na,S, 2.25% Na2CO; and 90.9% H2O. The sodium carbonate for precipitation was added in the form of anhydrous soda ash which contained CaCO3 as an impurity. Calculate:
Principles of Modern Chemistry
8th Edition
ISBN:9781305079113
Author:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Chapter15: Acid–base Equilibria
Section: Chapter Questions
Problem 98AP
Related questions
Question
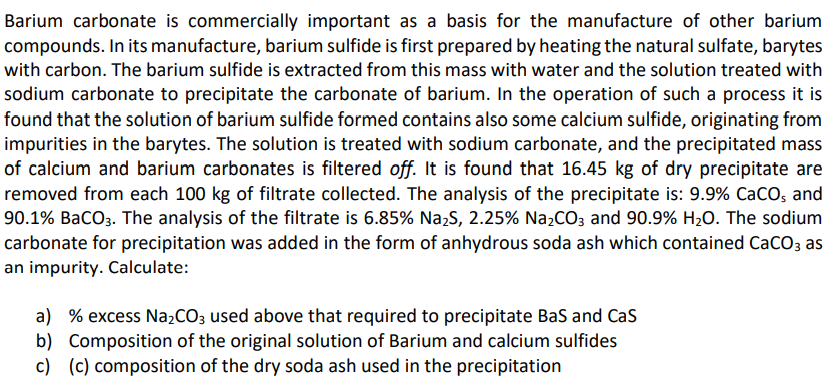
Transcribed Image Text:Barium carbonate is commercially important as a basis for the manufacture of other barium
compounds. In its manufacture, barium sulfide is first prepared by heating the natural sulfate, barytes
with carbon. The barium sulfide is extracted from this mass with water and the solution treated with
sodium carbonate to precipitate the carbonate of barium. In the operation of such a process it is
found that the solution of barium sulfide formed contains also some calcium sulfide, originating from
impurities in the barytes. The solution is treated with sodium carbonate, and the precipitated mass
of calcium and barium carbonates is filtered off. It is found that 16.45 kg of dry precipitate are
removed from each 100 kg of filtrate collected. The analysis of the precipitate is: 9.9% CaCO; and
90.1% BaCO3. The analysis of the filtrate is 6.85% Na,S, 2.25% Na2CO; and 90.9% H2O. The sodium
carbonate for precipitation was added in the form of anhydrous soda ash which contained CaCO; as
an impurity. Calculate:
a) % excess Na,CO; used above that required to precipitate Bas and Cas
b) Composition of the original solution of Barium and calcium sulfides
c) (c) composition of the dry soda ash used in the precipitation
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning