Based on the chart, solve the following questions with full solution: Mass to Mass Stoichiometry Calculation Limiting Reagent Excess Reagent Amount (g) in excess Percent (%) Yield Number of dosage form and packaging that can be produced from stoichiometric calculation
Based on the chart, solve the following questions with full solution: Mass to Mass Stoichiometry Calculation Limiting Reagent Excess Reagent Amount (g) in excess Percent (%) Yield Number of dosage form and packaging that can be produced from stoichiometric calculation
Chemistry for Engineering Students
4th Edition
ISBN:9781337398909
Author:Lawrence S. Brown, Tom Holme
Publisher:Lawrence S. Brown, Tom Holme
Chapter5: Gases
Section: Chapter Questions
Problem 5.3PAE: What possible uses exist for the natural gas liquids that are removed from natural gas during its...
Related questions
Question
Based on the chart, solve the following questions with full solution:
- Mass to Mass Stoichiometry Calculation
- Limiting Reagent
- Excess Reagent
- Amount (g) in excess
- Percent (%) Yield
- Number of dosage form and packaging that can be produced from stoichiometric calculation
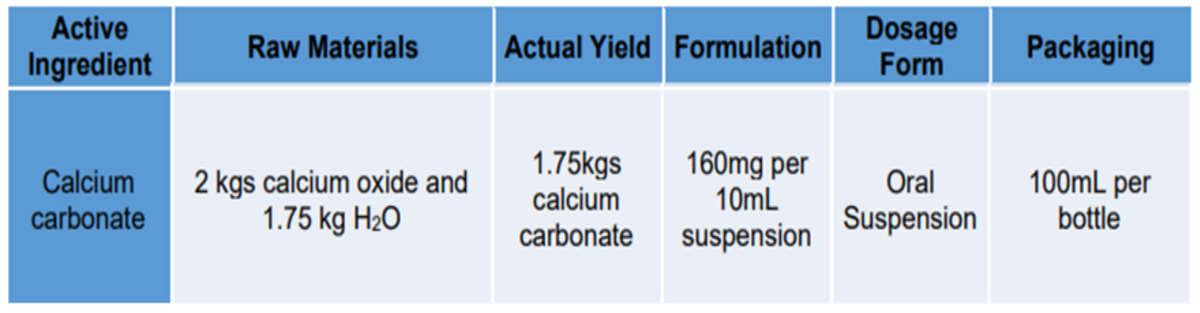
Transcribed Image Text:Dosage
Form
Active
Raw Materials
Actual Yield Formulation
Packaging
Ingredient
1.75kgs
calcium
carbonate
160mg per
10mL
100mL per
Calcium
carbonate
2 kgs calcium oxide and
1.75 kg H2O
Oral
Suspension
bottle
suspension
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning


Chemistry for Engineering Students
Chemistry
ISBN:
9781285199023
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning


Chemistry for Engineering Students
Chemistry
ISBN:
9781285199023
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning