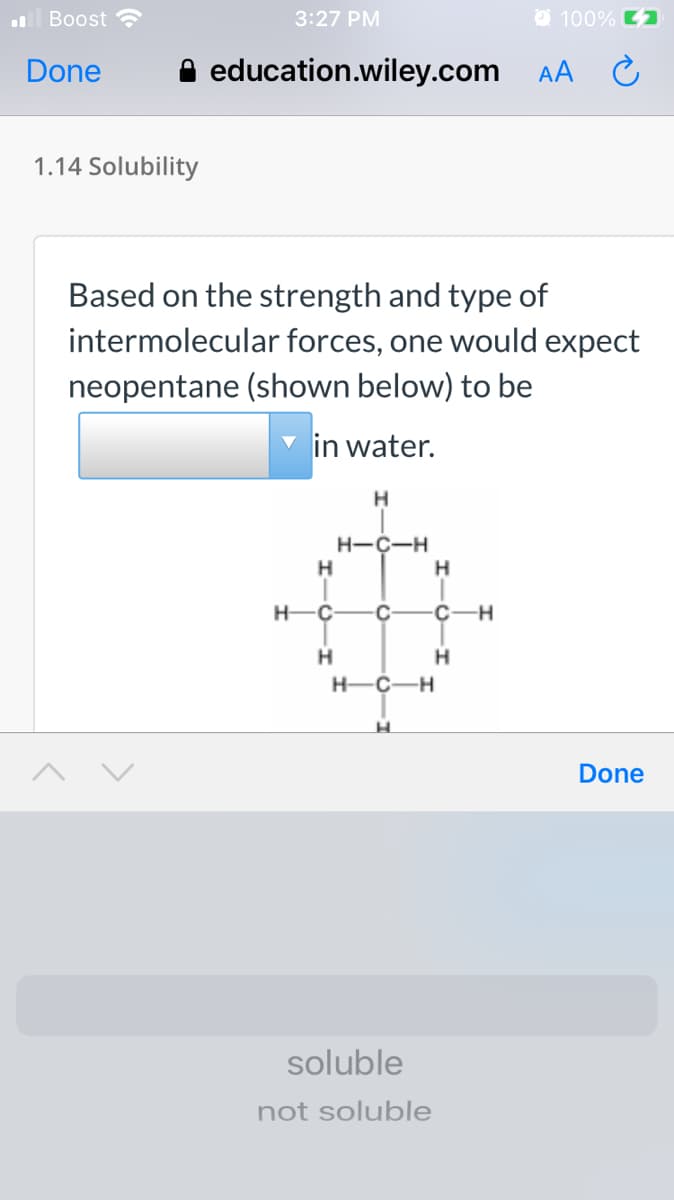
Based on the strength and type of intermolecular forces, one would expect neopentane (shown below) to be in water. H H-C-H H H-Ç -C- C-H H H-C-H
Based on the strength and type of intermolecular forces, one would expect neopentane (shown below) to be in water. H H-C-H H H-Ç -C- C-H H H-C-H
Chapter11: Properties Of Solutions
Section: Chapter Questions
Problem 47E: Rationalize the trend in water solubility for the following simple alcohols: Alcohol Solubility...
Related questions
Question
Transcribed Image Text:Boost
3:27 PM
O 100% Ca
Done
education.wiley.com AA C
1.14 Solubility
Based on the strength and type of
intermolecular forces, one would expect
neopentane (shown below) to be
in water.
H-C-H
H
H.
H-C-
-C-
C-H
H
H
H-C-H
Done
soluble
not soluble
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Recommended textbooks for you


Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax