HYDROGEN BONDING TO3/S04 Select all cases in which the liquid state of the pure substance exhibits hydrogen bonding. CHFCHF cis-1,2-difluoroethene CI3CCI3 hexachloroethane CH3COCH3CH2 methylethylketone (CH3)2CO acetone (CH3)3N trimethylamine CF4 carbon tetrafluoride None Question Attempts: 0 of 1 used SAVE FOR LATER SUBMIT ANSWER
HYDROGEN BONDING TO3/S04 Select all cases in which the liquid state of the pure substance exhibits hydrogen bonding. CHFCHF cis-1,2-difluoroethene CI3CCI3 hexachloroethane CH3COCH3CH2 methylethylketone (CH3)2CO acetone (CH3)3N trimethylamine CF4 carbon tetrafluoride None Question Attempts: 0 of 1 used SAVE FOR LATER SUBMIT ANSWER
Chemistry by OpenStax (2015-05-04)
1st Edition
ISBN:9781938168390
Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Chapter10: Liquids And Solids
Section: Chapter Questions
Problem 20E: The density of liquid NH3 is 0.64 g/mL; the density of gaseous NH3 at STP is 0.0007 g/mL. Explain...
Related questions
Question
please provide in depth explainations as I will be using this to study.
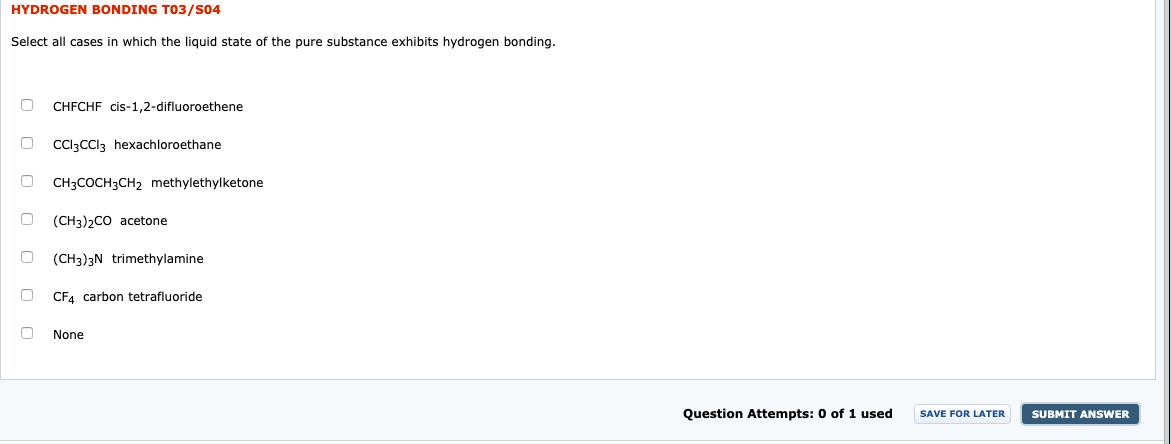
Transcribed Image Text:HYDROGEN BONDING TO3/S04
Select all cases in which the liquid state of the pure substance exhibits hydrogen bonding.
CHFCHF cis-1,2-difluoroethene
CI3CCI3 hexachloroethane
CH3COCH3CH2 methylethylketone
(CH3)2CO acetone
(CH3)3N trimethylamine
CF4 carbon tetrafluoride
None
Question Attempts: 0 of 1 used
SAVE FOR LATER
SUBMIT ANSWER
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 8 steps with 4 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning

General, Organic, and Biological Chemistry
Chemistry
ISBN:
9781285853918
Author:
H. Stephen Stoker
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning

General, Organic, and Biological Chemistry
Chemistry
ISBN:
9781285853918
Author:
H. Stephen Stoker
Publisher:
Cengage Learning