Based on the unit cell shown, what is the formula of the structure on the left (i.e. the one labeled “perovskite”)? Explain (in words or calculations) how you know.
Based on the unit cell shown, what is the formula of the structure on the left (i.e. the one labeled “perovskite”)? Explain (in words or calculations) how you know.
Chemistry by OpenStax (2015-05-04)
1st Edition
ISBN:9781938168390
Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Chapter19: Transition Metals And Coordination Chemistry
Section: Chapter Questions
Problem 8E: The following reactions all occur in a blast furnace. Which of these are redox reactions? (a)...
Related questions
Question
Based on the unit cell shown, what is the formula of the structure on the left (i.e. the one labeled “perovskite”)? Explain (in words or calculations) how you know.
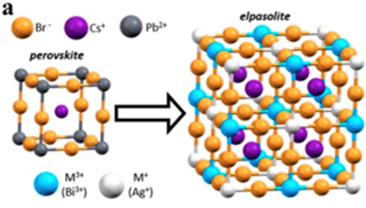
Transcribed Image Text:a
elpasolite
Br
Pb
рerovikite
M
(Bi")
M*
(Ag")
Expert Solution
Introduction
A unit cell of a crystal is the smallest repeating unit of space lattice from which the entire crystal may be build up purely translational displacements.
There are different types of space lattice , which are, cubic, body centered, face centred etc.
The no.of atoms present per unit cell will be different for each kind of lattice.
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning