Based on your proposed structure, how many moles of alcohol were used? b. What was the limiting reagent of the reaction? c. What is the theoretical yield of the ester? d. What is the percent yield?
Based on your proposed structure, how many moles of alcohol were used? b. What was the limiting reagent of the reaction? c. What is the theoretical yield of the ester? d. What is the percent yield?
Physical Chemistry
2nd Edition
ISBN:9781133958437
Author:Ball, David W. (david Warren), BAER, Tomas
Publisher:Ball, David W. (david Warren), BAER, Tomas
Chapter6: Equilibria In Single-component Systems
Section: Chapter Questions
Problem 6.25E: 6.25. Phosphorus exists as several allotropes that have varying properties. The enthalpy of...
Related questions
Question
a. Based on your proposed structure, how many moles of alcohol were used?
b. What was the limiting reagent of the reaction?
c. What is the theoretical yield of the ester?
d. What is the percent yield?
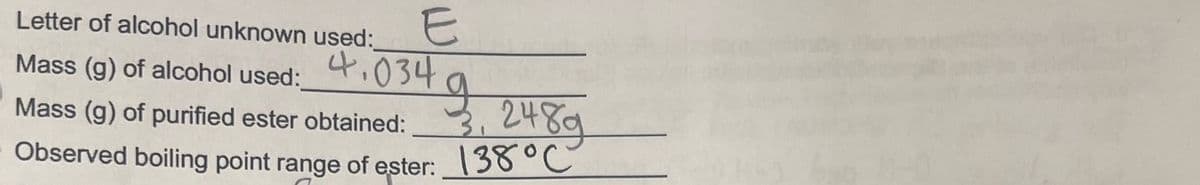
Transcribed Image Text:E
Letter of alcohol unknown used:
Mass (g) of alcohol used:_
g
3, 248g
Mass (g) of purified ester obtained:
Observed boiling point range of ester: 138 °C
4.034
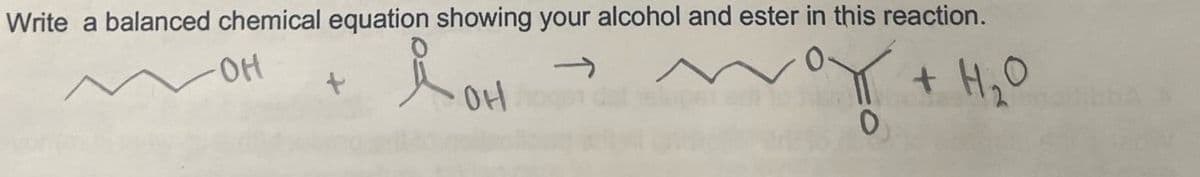
Transcribed Image Text:Write a balanced chemical equation showing your alcohol and ester in this reaction.
i OH
+ H2O
애
100
0
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax