Based your answer on the phase diagram of carbon dioxide a. What is the lowest temperature at which liquid carbon dioxide can exist? b. What is the critical temperature? c. What is the critical pressure? d. In what phase does CO2 exist at 20°C and 5.11 atm? 1.
Based your answer on the phase diagram of carbon dioxide a. What is the lowest temperature at which liquid carbon dioxide can exist? b. What is the critical temperature? c. What is the critical pressure? d. In what phase does CO2 exist at 20°C and 5.11 atm? 1.
Chemistry by OpenStax (2015-05-04)
1st Edition
ISBN:9781938168390
Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Chapter10: Liquids And Solids
Section: Chapter Questions
Problem 38E: Carbon tetrachloride, CCl4, was once used as a dry cleaning solvent, but is no longer used because...
Related questions
Question
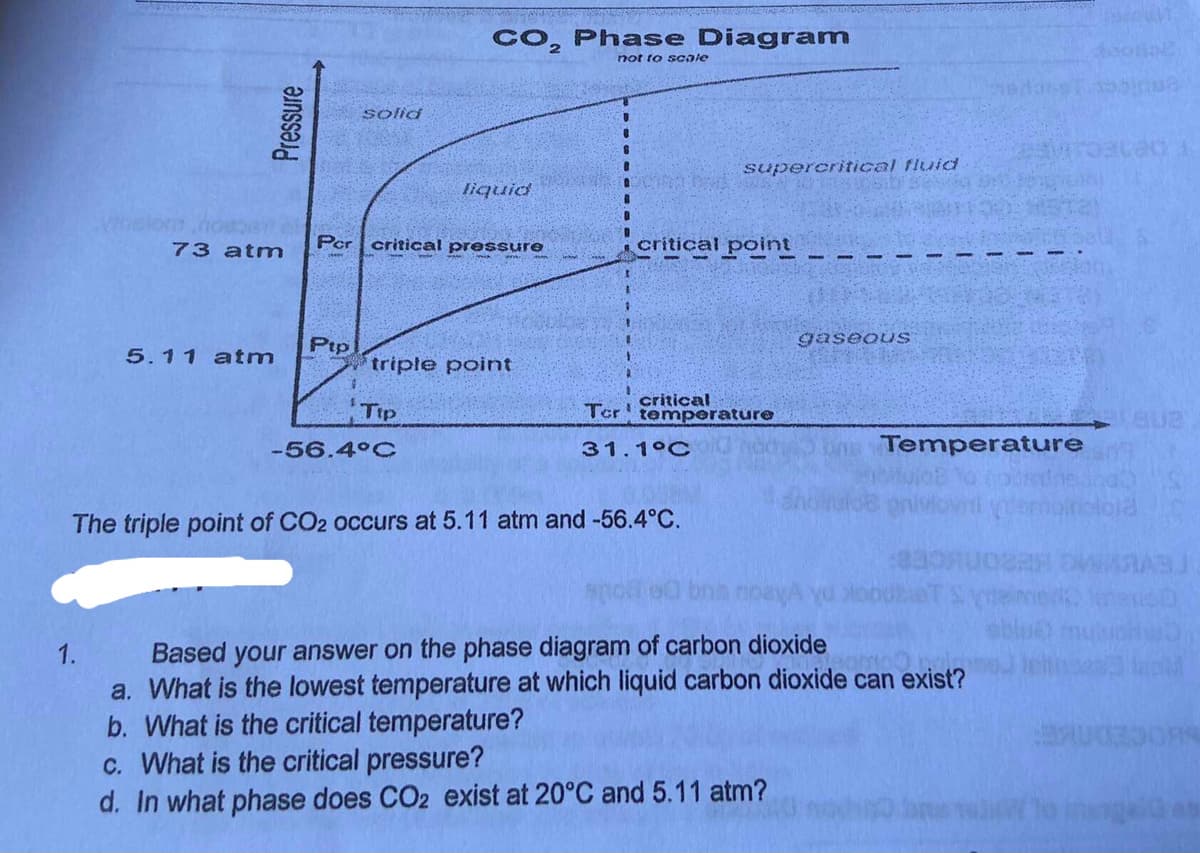
Transcribed Image Text:CO, Phase Diagram
not to scale
solid
supercritical fluid
liquid
Vihelora,ho
73 atm
Per critical pressure
critical point
gaseouS
Ptp
*triple point
5.11 atm
Tip
critical
Ter temperature
-56.4°C
31.1°C
Temperature
The triple point of CO2 occurs at 5.11 atm and -56.4°C.
Based your answer on the phase diagram of carbon dioxide
a. What is the lowest temperature at which liquid carbon dioxide can exist?
b. What is the critical temperature?
c. What is the critical pressure?
d. In what phase does CO2 exist at 20°C and 5.11 atm?
1.
ORS
Pressure
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning