1. The following diagram shows the partial synthesis of an important antihistamine drug currently used in the United States. One of the carbon atoms has been marked with an asterisk (*) to make it easy to spot as it undergoes structural changes from one product to the next. (a) In the box provided beneath each structure below, NEATLY write in the oxidation state for the starred carbon (*C) within that compound. Be sure to show your work in the space provided below. (b) Circle the appropriate label below each reaction arrow to indicate whether each step is an oxidation reaction, a reduction reaction, or neither.
1. The following diagram shows the partial synthesis of an important antihistamine drug currently used in the United States. One of the carbon atoms has been marked with an asterisk (*) to make it easy to spot as it undergoes structural changes from one product to the next. (a) In the box provided beneath each structure below, NEATLY write in the oxidation state for the starred carbon (*C) within that compound. Be sure to show your work in the space provided below. (b) Circle the appropriate label below each reaction arrow to indicate whether each step is an oxidation reaction, a reduction reaction, or neither.
Organic Chemistry
8th Edition
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Chapter22: Reactions Of Benzene And Its Derivatives
Section: Chapter Questions
Problem 22.51P
Related questions
Question
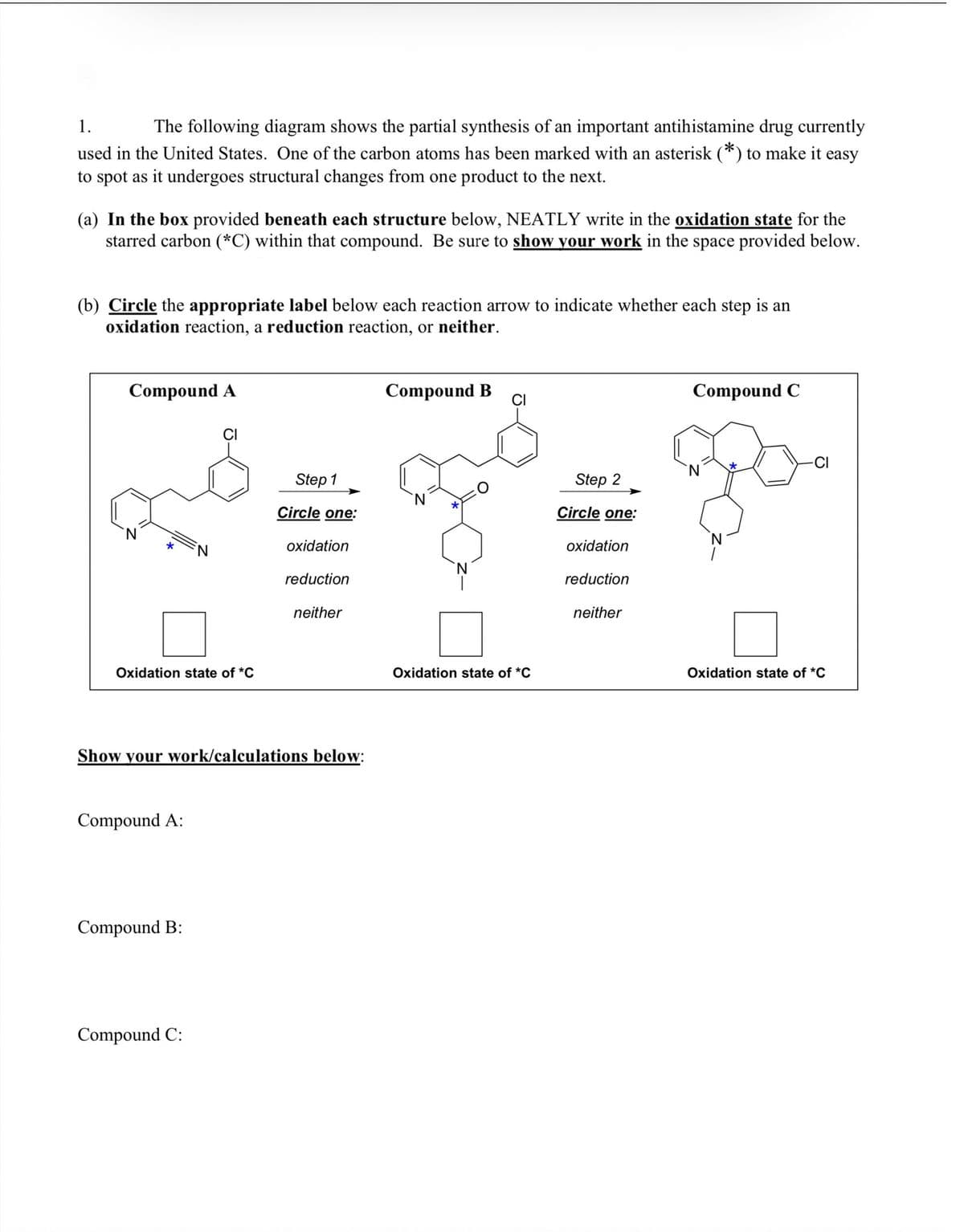
Transcribed Image Text:1.
The following diagram shows the partial synthesis of an important antihistamine drug currently
used in the United States. One of the carbon atoms has been marked with an asterisk (*) to make it easy
to spot as it undergoes structural changes from one product to the next.
(a) In the box provided beneath each structure below, NEATLY write in the oxidation state for the
starred carbon (*C) within that compound. Be sure to show your work in the space provided below.
(b) Circle the appropriate label below each reaction arrow to indicate whether each step is an
oxidation reaction, a reduction reaction, or neither.
Compound A
Compound B
Compound C
CI
CI
-CI
N.
Step 1
Step 2
Circle one:
Circle one:
N.
oxidation
oxidation
reduction
reduction
neither
neither
Oxidation state of *C
Oxidation state of *C
Oxidation state of *C
Show your work/calculations below:
Compound A:
Compound B:
Compound C:
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 3 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning

Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning