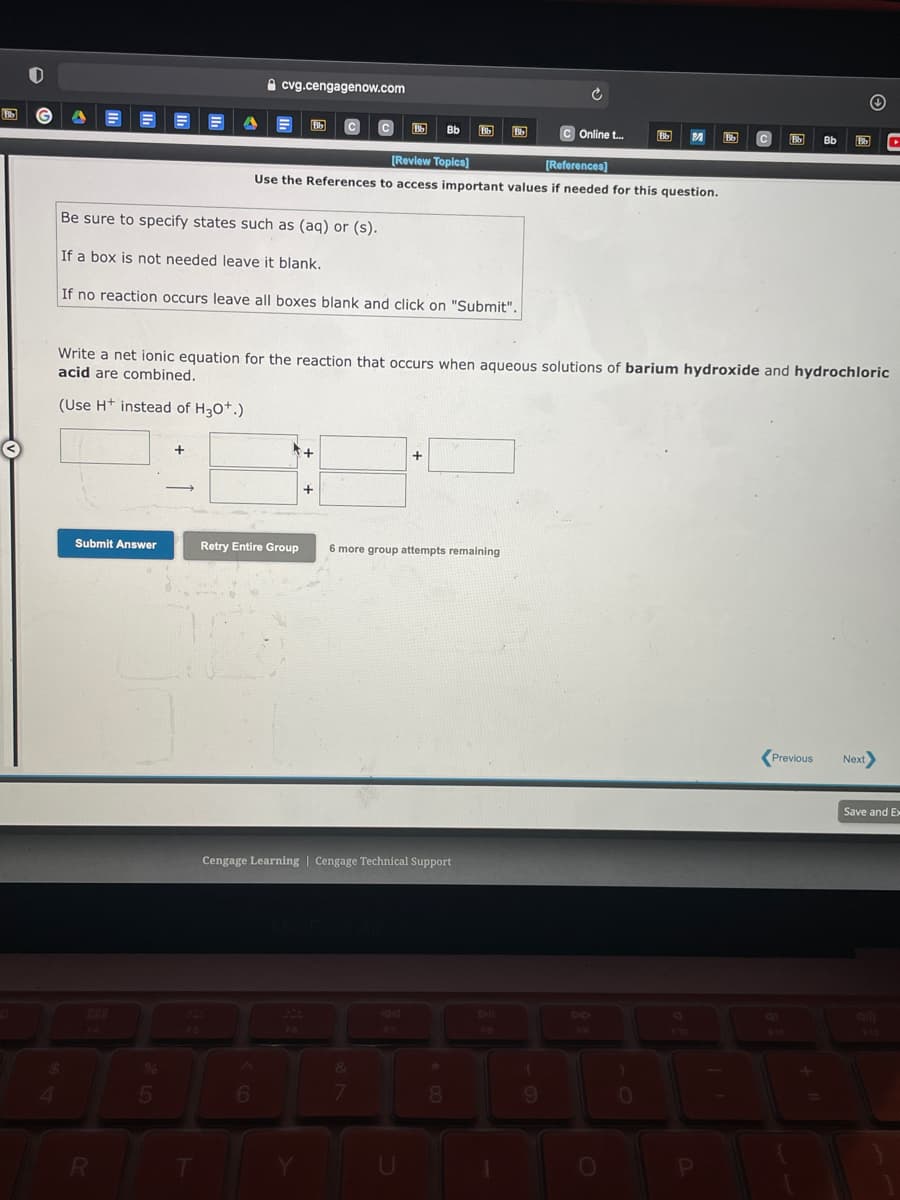
Bb C Online t. Bb (Review Topica) (References) Use the References to access important values if needed for this question. Be sure to specify states such as (aq) or (s). If a box is not needed leave it blank. If no reaction occurs leave all boxes blank and click on "Submit". Write a net ionic equation for the reaction that occurs when aqueous solutions of barium hydroxide and hydrochlo acid are combined. (Use H* instead of H30*.) + Submit Answer Retry Entire Group 6 more group attempts remaining
Bb C Online t. Bb (Review Topica) (References) Use the References to access important values if needed for this question. Be sure to specify states such as (aq) or (s). If a box is not needed leave it blank. If no reaction occurs leave all boxes blank and click on "Submit". Write a net ionic equation for the reaction that occurs when aqueous solutions of barium hydroxide and hydrochlo acid are combined. (Use H* instead of H30*.) + Submit Answer Retry Entire Group 6 more group attempts remaining
Chemical Principles in the Laboratory
11th Edition
ISBN:9781305264434
Author:Emil Slowinski, Wayne C. Wolsey, Robert Rossi
Publisher:Emil Slowinski, Wayne C. Wolsey, Robert Rossi
Chapter35: Spot Tests For Some Common Anions
Section: Chapter Questions
Problem 3ASA
Related questions
Question
Transcribed Image Text:A cvg.cengagenow.com
Bb
c Online t.
[Review Topics)
(References)
Use the References to access important values if needed for this question.
Be sure to specify states such as (aq) or (s).
If a box is not needed leave it blank.
If no reaction occurs leave all boxes blank and click on "Submit".
Write a net ionic equation for the reaction that occurs when aqueous solutions of barium hydroxide and hydrochloric
acid are combined.
(Use H+ instead of H30*.)
+
Submit Answer
Retry Entire Group
6 more group attempts remaining
Previous
Next
Save and Ex
Cengage Learning | Cengage Technical Support
F6
5
7.
080
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemical Principles in the Laboratory
Chemistry
ISBN:
9781305264434
Author:
Emil Slowinski, Wayne C. Wolsey, Robert Rossi
Publisher:
Brooks Cole

World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemical Principles in the Laboratory
Chemistry
ISBN:
9781305264434
Author:
Emil Slowinski, Wayne C. Wolsey, Robert Rossi
Publisher:
Brooks Cole

World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:
9780078746376
Author:
Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:
Glencoe/McGraw-Hill School Pub Co

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning