be shipped as a liquid. Suppose 2.62 × 10° ft of methane is oxidized to methanol at 1.00 atm and 25 °C. What volume of methanol, in liters, is formed? The density of methanol is 0.791 g/mL. volume: Write the balanced chemical equations for the combustion of methane gas and liquid methanol to CO, (g) and H, O(1). Include phases.
be shipped as a liquid. Suppose 2.62 × 10° ft of methane is oxidized to methanol at 1.00 atm and 25 °C. What volume of methanol, in liters, is formed? The density of methanol is 0.791 g/mL. volume: Write the balanced chemical equations for the combustion of methane gas and liquid methanol to CO, (g) and H, O(1). Include phases.
Chemistry: The Molecular Science
5th Edition
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:John W. Moore, Conrad L. Stanitski
Chapter4: Energy And Chemical Reactions
Section: Chapter Questions
Problem 122QRT
Related questions
Question
How do I solve the following?
Transcribed Image Text:O Resources
Give Uo
O Hint
Check Answer
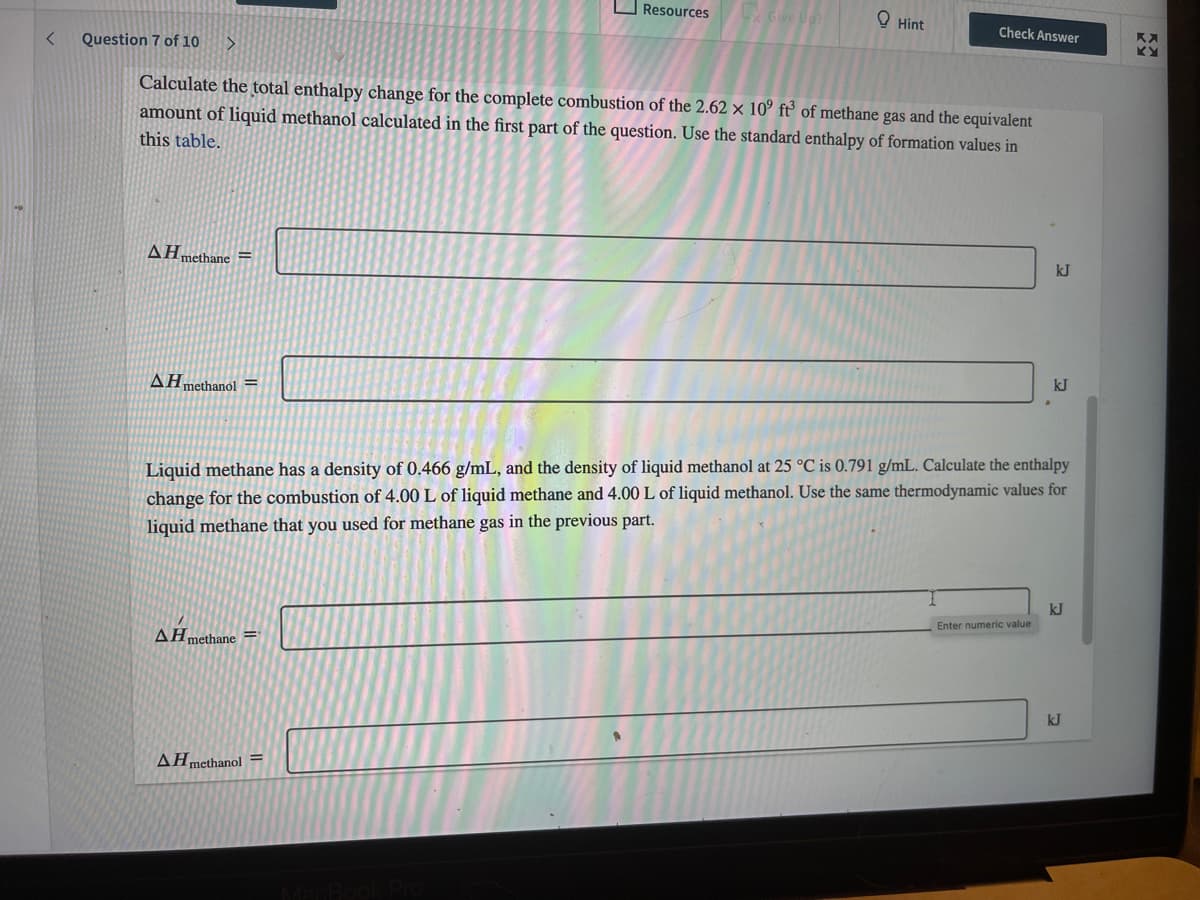
Question 7 of 10
Calculate the total enthalpy change for the complete combustion of the 2.62 × 10° f° of methane gas and the equivalent
amount of liquid methanol calculated in the first part of the question. Use the standard enthalpy of formation values in
this table.
AHmethane =
kJ
kJ
AHmethanol =
Liquid methane has a density of 0.466 g/mL, and the density of liquid methanol at 25 °C is 0.791 g/mL. Calculate the enthalpy
change for the combustion of 4.00 L of liquid methane and 4.00 L of liquid methanol. Use the same thermodynamic values for
liquid methane that you used for methane gas in the previous part.
kJ
Enter numeric value
AHmethane =
kJ
AHmethanol =

Transcribed Image Text:Many wells drilled in the United States produce both oil and natural gas. In the past, the natural gas was treated as a waste
byproduct of oil production and was burned off because the cost of shipping the gas was prohibitively high since it is
necessary to liquefy the gas, which is mainly methane (CH) and has a boiling point of –164 °C. As the natural gas market has
grown, liquefied natural gas (LNG) carriers, tank ships designed for transporting LNG, have become more prevalent and the
shipping costs have decreased. Before the rise in LNG tankers, one strategy used for transporting natural gas was to oxidize the
methane to methanol (CH,OH), which has a boiling point of 65 °C, and can more readily be shipped as a liquid.
Suppose 2.62 x 10° ft of methane is oxidized to methanol at 1.00 atm and 25 °C. What volume of methanol, in liters, is
formed? The density of methanol is 0.791 g/mL.
volume:
L.
Write the balanced chemical equations for the combustion of methane gas and liquid methanol to CO, (g) and H,O(1).
Include phases.
combustion of methane:
combustion of methanol:
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 4 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax