Be sure to answer all parts. A solution of 2.01 g of anhydrous aluminum chloride (AlCl3) in 54.46 g of wat Calculate the molar mass of AICI3 from the formula. 33.32 g/mol Calculate the molar mass from the freezing point depression, assuming AlCl3 g/mol Comparing the two values, how many ions will AlCl3 form?. O 1 O2 O 3 4
Be sure to answer all parts. A solution of 2.01 g of anhydrous aluminum chloride (AlCl3) in 54.46 g of wat Calculate the molar mass of AICI3 from the formula. 33.32 g/mol Calculate the molar mass from the freezing point depression, assuming AlCl3 g/mol Comparing the two values, how many ions will AlCl3 form?. O 1 O2 O 3 4
Chemistry: Matter and Change
1st Edition
ISBN:9780078746376
Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Chapter14: Mixtures And Solutions
Section14.4: Colligative Properties Of Solutions
Problem 47PP
Related questions
Question
100%
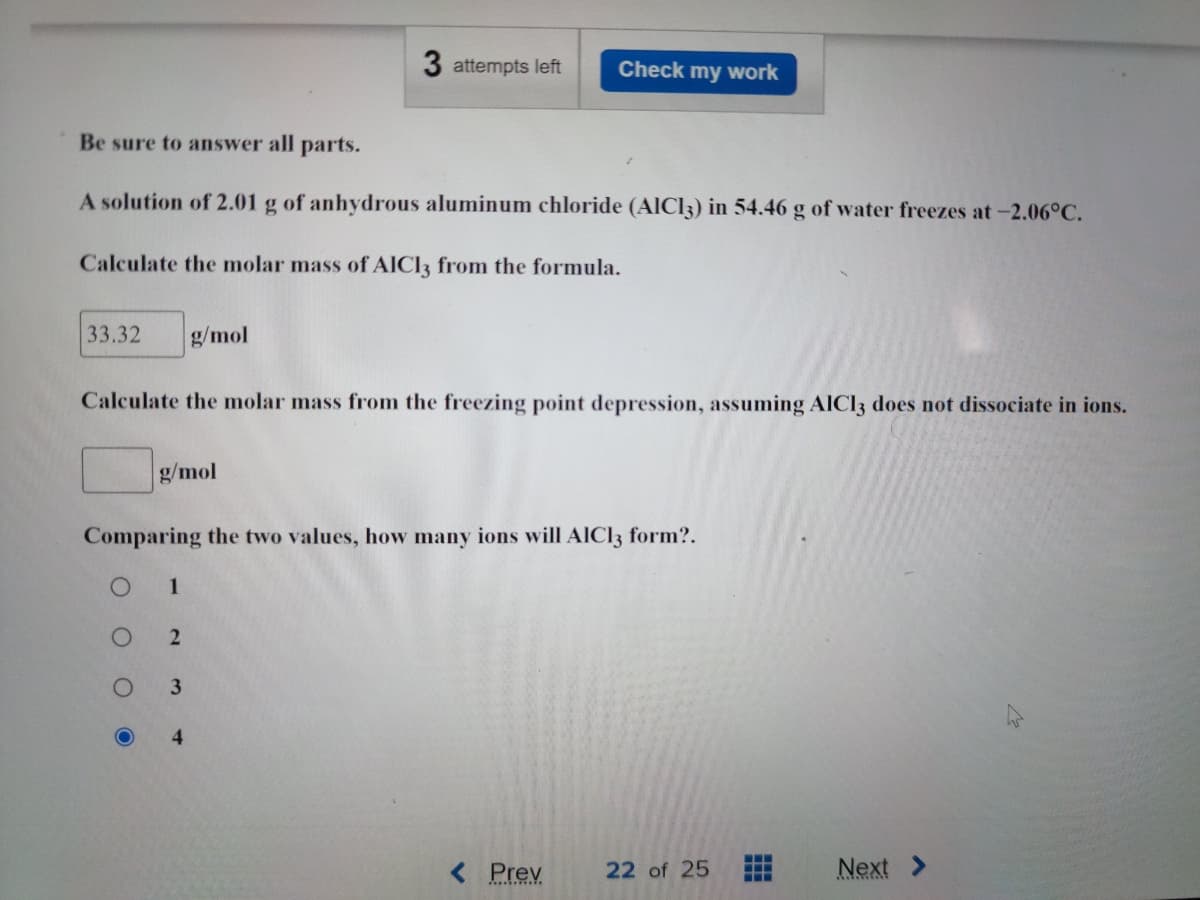
Transcribed Image Text:3 attempts left Check my work
Be sure to answer all parts.
A solution of 2.01 g of anhydrous aluminum chloride (AlCl3) in 54.46 g of water freezes at -2.06°C.
Calculate the molar mass of AICI3 from the formula.
33.32 g/mol
Calculate the molar mass from the freezing point depression, assuming AlCl3 does not dissociate in ions.
g/mol
Comparing the two values, how many ions will AlCl3 form?.
1
2
3
4
< Prev 22 of 25
Next >
O
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 3 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: Matter and Change
Chemistry
ISBN:
9780078746376
Author:
Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:
Glencoe/McGraw-Hill School Pub Co

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:
9780078746376
Author:
Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:
Glencoe/McGraw-Hill School Pub Co

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning