Be sure to answer all parts. Consider the following balanced thermochemical equation for the decomposition of the mineral magnesite: MgCO,)MgO)+ CO,) AH-117.3 J (a) Is heat absorbed or released in the reaction? absorbed released (b) What is AH for the reverse reaction? kJ (e) What is AH when 5.20 mol of CO, reacts with excess Mg0? kJ (d) What is AH when 32.5 g of CO, reacts with excess Mg0? kJ
Be sure to answer all parts. Consider the following balanced thermochemical equation for the decomposition of the mineral magnesite: MgCO,)MgO)+ CO,) AH-117.3 J (a) Is heat absorbed or released in the reaction? absorbed released (b) What is AH for the reverse reaction? kJ (e) What is AH when 5.20 mol of CO, reacts with excess Mg0? kJ (d) What is AH when 32.5 g of CO, reacts with excess Mg0? kJ
General Chemistry - Standalone book (MindTap Course List)
11th Edition
ISBN:9781305580343
Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Chapter6: Thermochemisty
Section: Chapter Questions
Problem 6.132QP
Related questions
Question
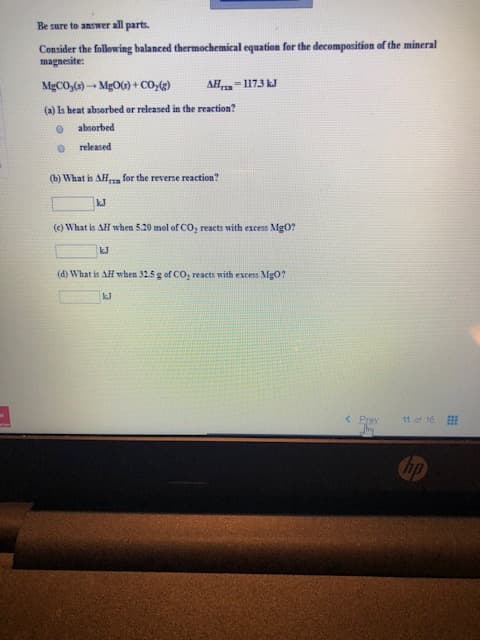
Transcribed Image Text:Be sure to answer all parts.
Consider the following balanced thermochemical equation for the decomposition of the mineral
magnesite:
MgCO,)MgO)+ CO,)
AH-117.3 J
(a) Is heat absorbed or released in the reaction?
absorbed
released
(b) What is AH
for the reverse reaction?
kJ
(e) What is AH when 5.20 mol of CO, reacts with excess Mg0?
kJ
(d) What is AH when 32.5 g of CO, reacts with excess Mg0?
kJ
<Prev
11 of 16
hp
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 1 images

Recommended textbooks for you

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781285199023
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning