Below is the resolved absorption spectrum of HBr for the vibrational transition v = 0 → V = 1; determine: (a) the effective spring constant for the molecular bond 35 and (b) the bond length. (c) Repeat for both the HCl 37 and H Cl spectra. (d) Based upon these results, explain your prediction regarding which molecule has the "strongest" bond and then explain whether your prediction is confirmed by looking up the corresponding bond dissociation energies. ✓ 2 0.300 0.310 0.320 0.330 0.340 Energy (eV)
Below is the resolved absorption spectrum of HBr for the vibrational transition v = 0 → V = 1; determine: (a) the effective spring constant for the molecular bond 35 and (b) the bond length. (c) Repeat for both the HCl 37 and H Cl spectra. (d) Based upon these results, explain your prediction regarding which molecule has the "strongest" bond and then explain whether your prediction is confirmed by looking up the corresponding bond dissociation energies. ✓ 2 0.300 0.310 0.320 0.330 0.340 Energy (eV)
Principles of Instrumental Analysis
7th Edition
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Chapter9: Atomic Absorption And Atomic Fluorescence Spectrometry
Section: Chapter Questions
Problem 9.8QAP
Related questions
Question
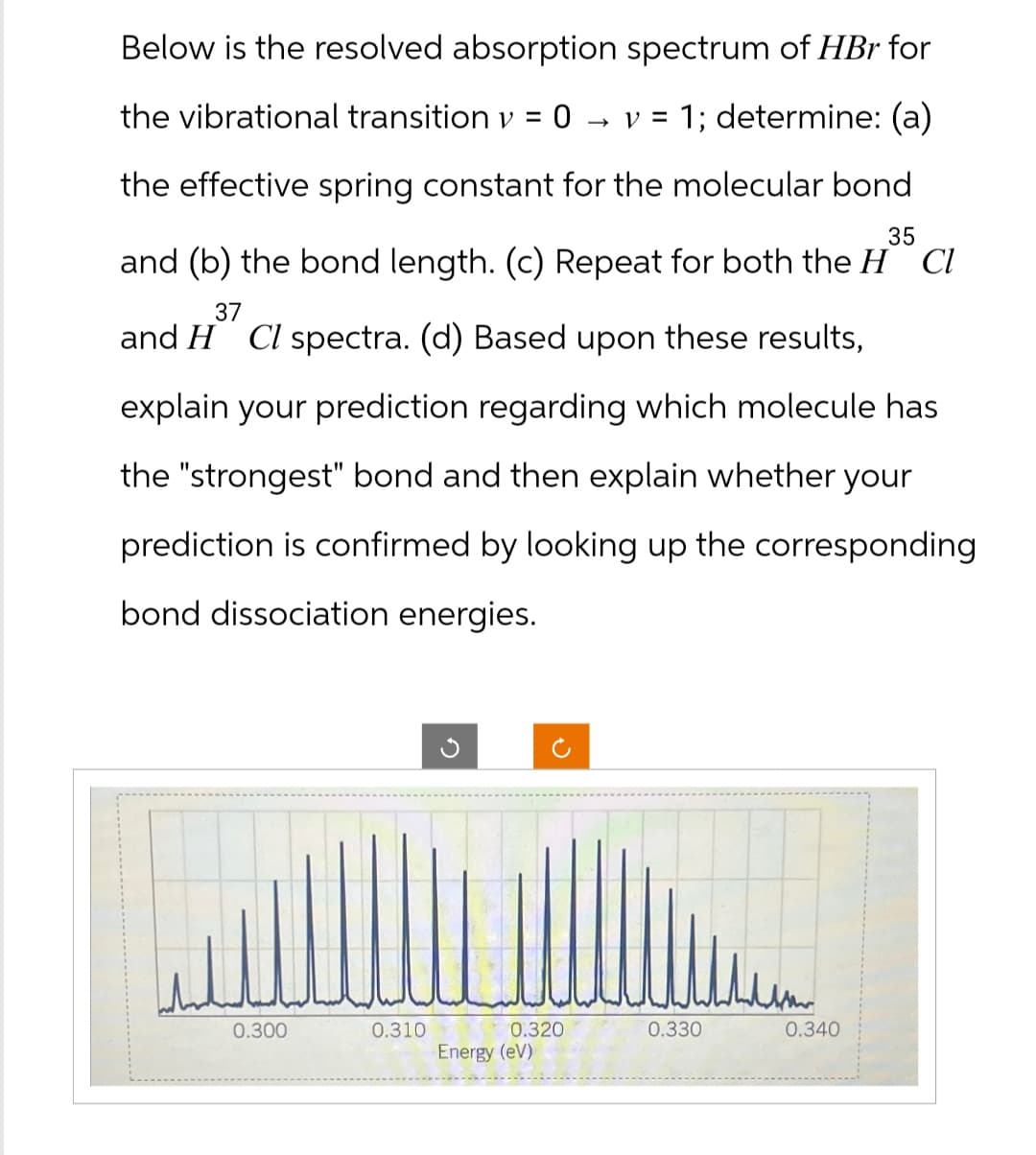
Transcribed Image Text:Below is the resolved absorption spectrum of HBr for
the vibrational transition v = 0
→ V = 1; determine: (a)
the effective spring constant for the molecular bond
35
and (b) the bond length. (c) Repeat for both the HCl
37
and H Cl spectra. (d) Based upon these results,
explain your prediction regarding which molecule has
the "strongest" bond and then explain whether your
prediction is confirmed by looking up the corresponding
bond dissociation energies.
✓
2
0.300
0.310
0.320
0.330
0.340
Energy (eV)
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps

Recommended textbooks for you

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning