MODEL 10.1 A (Reactant) - B- A AG: CHANGE IN FREE ENERGY Energy available to do work Approaches zero as reaction proceeds to equillibrium • Predicts whether a reaction is favorable (Product) Transition state Transition state AH: CHANGE IN ENTHALPY • Heat released or absorbed during a reaction • Does not predict whether a reaction is favorable A Initial state Final state AG is negative AG is positive Change in free energy of reaction AG = AH-TAS %3D AS: CHANGE IN ENTROPY Initial state Final st Measure of randomness • Does not predict whether a reaction is favorable Progress of reaction- Progress of reaction– B (5) KBJoue 00 Free energy (G) 1. What is free energy? What is its symbol? 3. For an endergonic, what is the value of AG? 2. For an exergonic reaction, what is the value of AG? 2. For an endergonic, what is the value of AG? 4 What are the factors that affect AG? 5. What is energy coupling? In a coupling reaction, what must be the overall value of AG? 6. What does the cell do with the energy produced from exergonic reactions? 7. What molecule does the cell use as an energy carrier? Draw its structure. 8. Why is it that this energy carrier is considered to be high energy containing phosphate? 9. Bond of this energy carrier of cells is broken through what? 2019. All Rights Reserved.
MODEL 10.1 A (Reactant) - B- A AG: CHANGE IN FREE ENERGY Energy available to do work Approaches zero as reaction proceeds to equillibrium • Predicts whether a reaction is favorable (Product) Transition state Transition state AH: CHANGE IN ENTHALPY • Heat released or absorbed during a reaction • Does not predict whether a reaction is favorable A Initial state Final state AG is negative AG is positive Change in free energy of reaction AG = AH-TAS %3D AS: CHANGE IN ENTROPY Initial state Final st Measure of randomness • Does not predict whether a reaction is favorable Progress of reaction- Progress of reaction– B (5) KBJoue 00 Free energy (G) 1. What is free energy? What is its symbol? 3. For an endergonic, what is the value of AG? 2. For an exergonic reaction, what is the value of AG? 2. For an endergonic, what is the value of AG? 4 What are the factors that affect AG? 5. What is energy coupling? In a coupling reaction, what must be the overall value of AG? 6. What does the cell do with the energy produced from exergonic reactions? 7. What molecule does the cell use as an energy carrier? Draw its structure. 8. Why is it that this energy carrier is considered to be high energy containing phosphate? 9. Bond of this energy carrier of cells is broken through what? 2019. All Rights Reserved.
Biology (MindTap Course List)
11th Edition
ISBN:9781337392938
Author:Eldra Solomon, Charles Martin, Diana W. Martin, Linda R. Berg
Publisher:Eldra Solomon, Charles Martin, Diana W. Martin, Linda R. Berg
Chapter7: Energy And Metabolism
Section: Chapter Questions
Problem 15TYU
Related questions
Question
please help me identify these huhu thank you
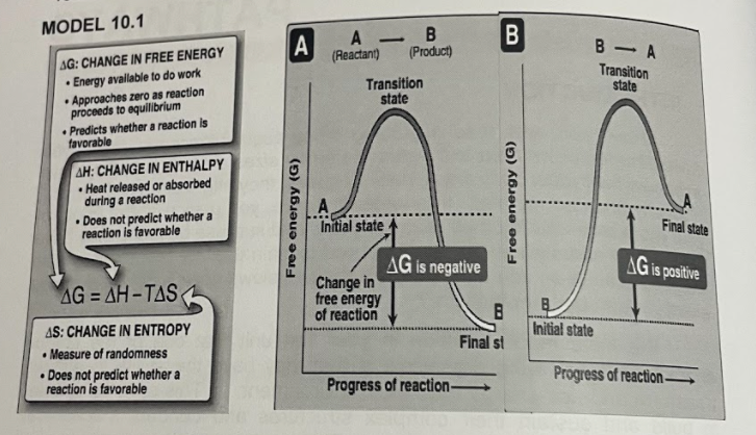
Transcribed Image Text:MODEL 10.1
A
(Reactant)
-
B- A
AG: CHANGE IN FREE ENERGY
Energy available to do work
Approaches zero as reaction
proceeds to equillibrium
• Predicts whether a reaction is
favorable
(Product)
Transition
state
Transition
state
AH: CHANGE IN ENTHALPY
• Heat released or absorbed
during a reaction
• Does not predict whether a
reaction is favorable
A
Initial state
Final state
AG is negative
AG is positive
Change in
free energy
of reaction
AG = AH-TAS
%3D
AS: CHANGE IN ENTROPY
Initial state
Final st
Measure of randomness
• Does not predict whether a
reaction is favorable
Progress of reaction-
Progress of reaction–
B
(5) KBJoue 00
Free energy (G)

Transcribed Image Text:1. What is free energy? What is its symbol?
3. For an endergonic, what is the value of AG?
2. For an exergonic reaction, what is the value of AG?
2. For an endergonic, what is the value of AG?
4 What are the factors that affect AG?
5. What is energy coupling? In a coupling reaction, what must be the
overall value of AG?
6. What does the cell do with the energy produced from exergonic
reactions?
7. What molecule does the cell use as an energy carrier? Draw its
structure.
8. Why is it that this energy carrier is considered to be high energy
containing phosphate?
9. Bond of this energy carrier of cells is broken through what?
2019. All Rights Reserved.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps

Recommended textbooks for you

Biology (MindTap Course List)
Biology
ISBN:
9781337392938
Author:
Eldra Solomon, Charles Martin, Diana W. Martin, Linda R. Berg
Publisher:
Cengage Learning

Biology 2e
Biology
ISBN:
9781947172517
Author:
Matthew Douglas, Jung Choi, Mary Ann Clark
Publisher:
OpenStax

Biology (MindTap Course List)
Biology
ISBN:
9781337392938
Author:
Eldra Solomon, Charles Martin, Diana W. Martin, Linda R. Berg
Publisher:
Cengage Learning

Biology 2e
Biology
ISBN:
9781947172517
Author:
Matthew Douglas, Jung Choi, Mary Ann Clark
Publisher:
OpenStax