blackboard.stlcc.edu Tes... Welcome To... Take Test: T... Sign out Si Remaining Time: 1 hour, 07 minutes, 10 seconds. ¥ Question Completion Status: H2X is the base and HX is the conjugate acid QUESTION 12 Which of the following could not be a Bronsted- Lowry Acid? O NH4 O NH3 О Н20 BЕЗ QUESTION 13 What volume of 0.0347 M Ba(OH)2 is needed to completely react wit 2 HC + Ba(OН)2 — ВаСl2 + 2 H20
blackboard.stlcc.edu Tes... Welcome To... Take Test: T... Sign out Si Remaining Time: 1 hour, 07 minutes, 10 seconds. ¥ Question Completion Status: H2X is the base and HX is the conjugate acid QUESTION 12 Which of the following could not be a Bronsted- Lowry Acid? O NH4 O NH3 О Н20 BЕЗ QUESTION 13 What volume of 0.0347 M Ba(OH)2 is needed to completely react wit 2 HC + Ba(OН)2 — ВаСl2 + 2 H20
Chapter30: Resolution Of (6)-a-phenylethylamine And Determination Of Optical Purity
Section: Chapter Questions
Problem 1Q
Related questions
Question
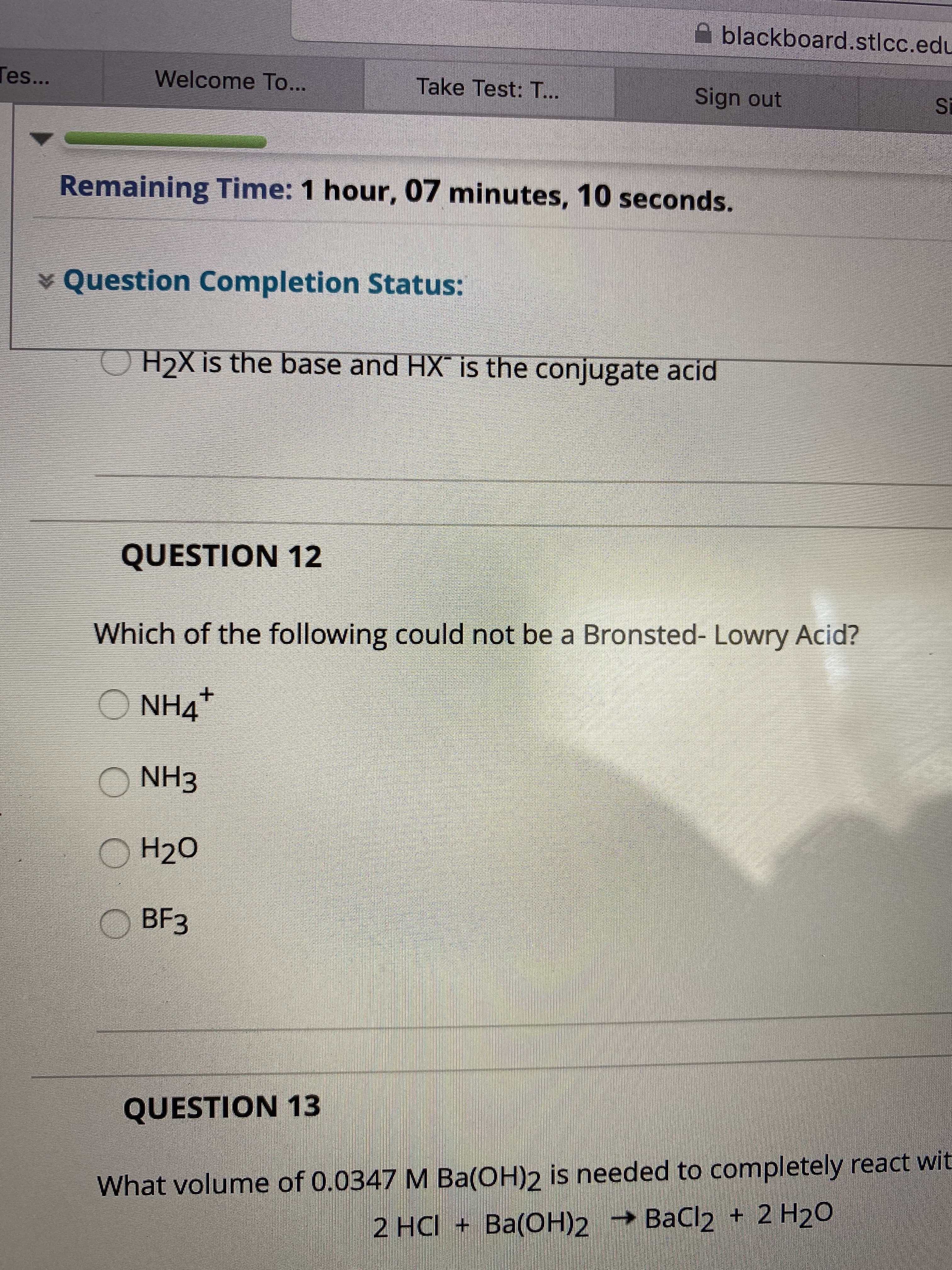
Transcribed Image Text:blackboard.stlcc.edu
Tes...
Welcome To...
Take Test: T...
Sign out
Si
Remaining Time: 1 hour, 07 minutes, 10 seconds.
¥ Question Completion Status:
H2X is the base and HX is the conjugate acid
QUESTION 12
Which of the following could not be a Bronsted- Lowry Acid?
O NH4
O NH3
О Н20
BЕЗ
QUESTION 13
What volume of 0.0347 M Ba(OH)2 is needed to completely react wit
2 HC + Ba(OН)2
— ВаСl2 + 2 H20
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 3 images

Recommended textbooks for you

EBK A SMALL SCALE APPROACH TO ORGANIC L
Chemistry
ISBN:
9781305446021
Author:
Lampman
Publisher:
CENGAGE LEARNING - CONSIGNMENT


EBK A SMALL SCALE APPROACH TO ORGANIC L
Chemistry
ISBN:
9781305446021
Author:
Lampman
Publisher:
CENGAGE LEARNING - CONSIGNMENT
