Blast furnaces extract pure iron from the iron(III) oxide in iron ore in a two step sequence. In the first step, carbon and oxygen react to form carbon monoxide: 2 C(s)+O,(g)→2CO(g) In the second step, iron(III) oxide and carbon monoxide react to form iron and carbon dioxide: Fe,0,(s)+3 CO(g)→2 Fe(s)+3 CO,(g) Write the net chemical equation for the production of iron from carbon, oxygen and iron(II) oxide. Be sure your equation is balanced. O-0
Blast furnaces extract pure iron from the iron(III) oxide in iron ore in a two step sequence. In the first step, carbon and oxygen react to form carbon monoxide: 2 C(s)+O,(g)→2CO(g) In the second step, iron(III) oxide and carbon monoxide react to form iron and carbon dioxide: Fe,0,(s)+3 CO(g)→2 Fe(s)+3 CO,(g) Write the net chemical equation for the production of iron from carbon, oxygen and iron(II) oxide. Be sure your equation is balanced. O-0
Chemistry: The Molecular Science
5th Edition
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:John W. Moore, Conrad L. Stanitski
Chapter3: Chemical Reactions
Section: Chapter Questions
Problem 122QRT: Carbon monoxide burns readily in oxygen to form carbon dioxide. 2 CO(g) + O2(g) 2 CO2(g) The box on...
Related questions
Question
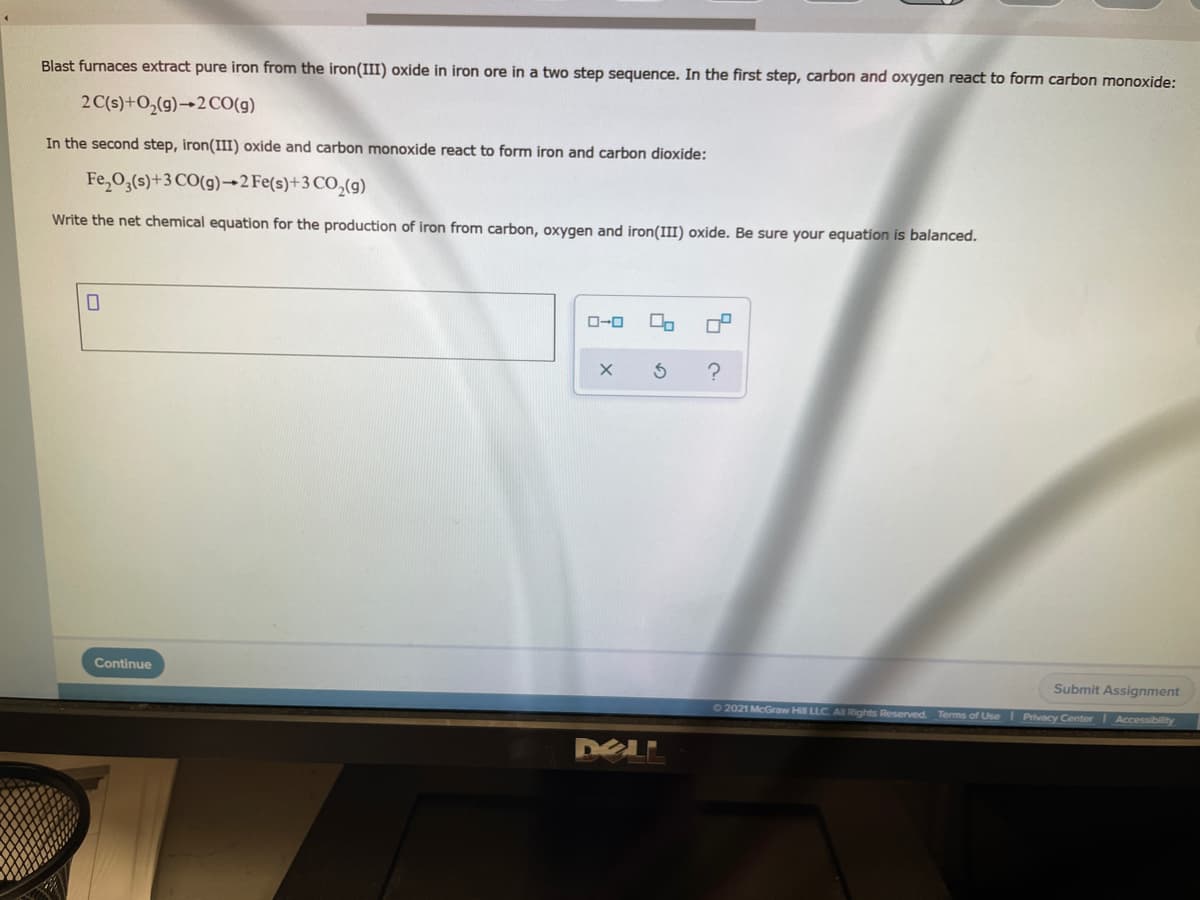
Transcribed Image Text:Blast furnaces extract pure iron from the iron(III) oxide in iron ore in a two step sequence. In the first step, carbon and oxygen react to form carbon monoxide:
2 C(s)+O,(g)→2CO(g)
In the second step, iron(III) oxide and carbon monoxide react to form iron and carbon dioxide:
Fe,03(s)+3 CO(g)→2 Fe(s)+3 CO,(g)
Write the net chemical equation for the production of iron from carbon, oxygen and iron(II) oxide. Be sure your equation is balanced.
O-0
Continue
Submit Assignment
O2021 McGraw Hill LLC A Rights Reserved.
Terms of Use Privacy Center Accessibility
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Recommended textbooks for you

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

General, Organic, and Biological Chemistry
Chemistry
ISBN:
9781285853918
Author:
H. Stephen Stoker
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning