Nitric acid is often manufactured from the atmospheric gases nitrogen and oxygen, plus hydrogen prepared by reforming of natural gas, in a two-step process. In the first step, nitrogen and hydrogen react to form ammonia: N,(9)+3 H,(g)→2 NH,(g) In the second step, ammonia and oxygen react to form nitric acid and water: NH,(g)+20,(g)→HNO,(g)+H,O(g) Write the net chemical equation for the production of nitric acid from nitrogen, hydrogen and oxygen. Be sure your equation is balanced.
Nitric acid is often manufactured from the atmospheric gases nitrogen and oxygen, plus hydrogen prepared by reforming of natural gas, in a two-step process. In the first step, nitrogen and hydrogen react to form ammonia: N,(9)+3 H,(g)→2 NH,(g) In the second step, ammonia and oxygen react to form nitric acid and water: NH,(g)+20,(g)→HNO,(g)+H,O(g) Write the net chemical equation for the production of nitric acid from nitrogen, hydrogen and oxygen. Be sure your equation is balanced.
Chemistry & Chemical Reactivity
10th Edition
ISBN:9781337399074
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Chapter3: Chemical Reactions
Section3.2: Balancing Chemical Equations
Problem 3.1CYU: (a) Butane gas, C4H10, can burn completely in air [use O2(g) as the other reactant] to give carbon...
Related questions
Question
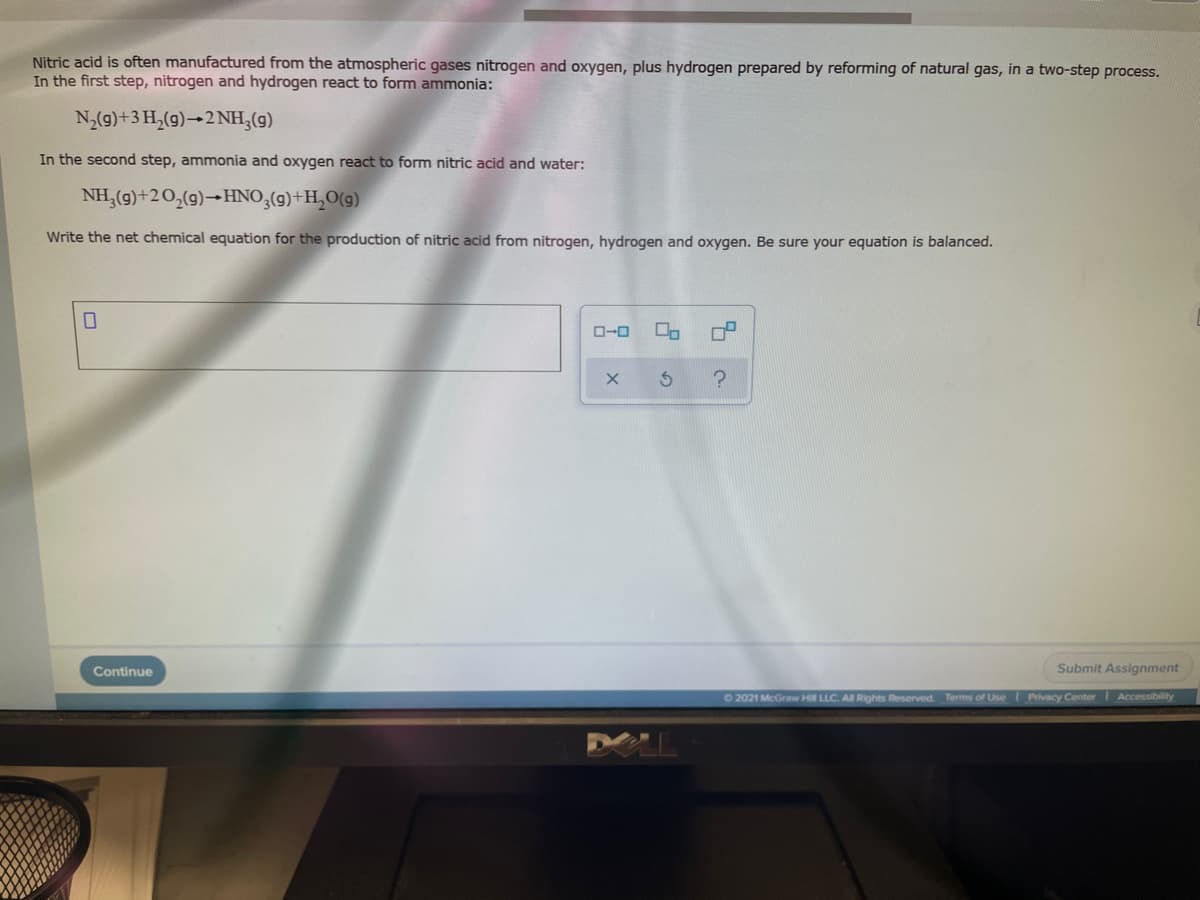
Transcribed Image Text:Nitric acid is often manufactured from the atmospheric gases nitrogen and oxygen, plus hydrogen prepared by reforming of natural gas, in a two-step process.
In the first step, nitrogen and hydrogen react to form ammonia:
N,(9)+3 H,(9)→2NH,(g)
In the second step, ammonia and oxygen react to form nitric acid and water:
NH,(g)+20,(g)-HNO,(g)+H,O(g)
Write the net chemical equation for the production of nitric acid from nitrogen, hydrogen and oxygen. Be sure your equation is balanced.
O-0
Continue
Submit Assignment
22021 McGravw Hill LLC. A Rights Reserved. Terms of Use
Privacy Center Accessibility
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Recommended textbooks for you

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781285199023
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781285199023
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning