Principles of Instrumental Analysis
7th Edition
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Chapter9: Atomic Absorption And Atomic Fluorescence Spectrometry
Section: Chapter Questions
Problem 9.10QAP
Related questions
Question
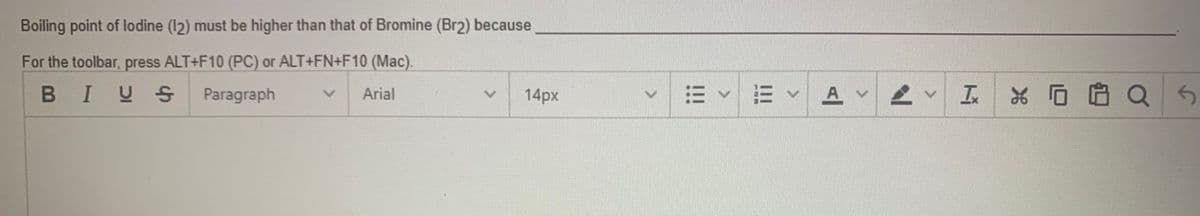
Transcribed Image Text:Boiling point of lodine (12) must be higher than that of Bromine (Br2) because
For the toolbar, press ALT+F10 (PC) or ALT+FN+F10 (Mac).
BIUS
1=vEvA<2I%0白Q
Paragraph
Arial
14px
Expert Solution
Step 1
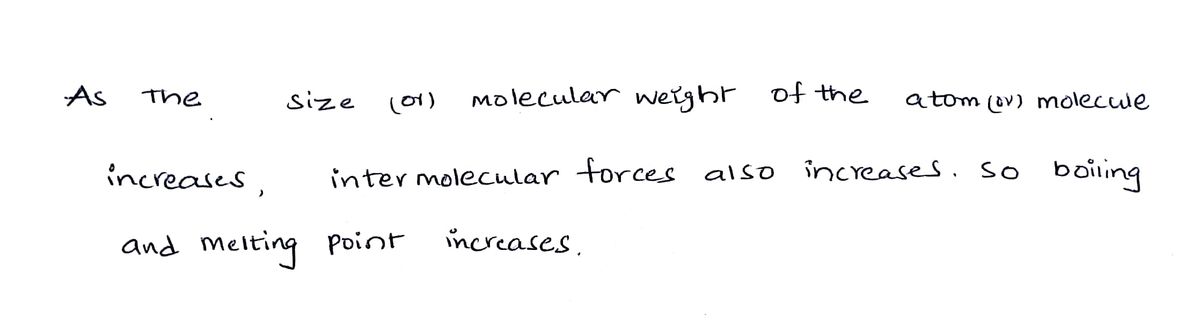
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning


Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning
