Both lithium (Li) and silver (Ag) have 2 naturally occurring isotopes. Calculate the atomic mass of each element using the given data for the % relative abundance and mass of each isotope. A) 1st isotope: Li-6 has % relative abundance = 7.42% and mass = 6.01 amu 2nd isotope: Li-7 has % relative abundance = 92.58% and mass = 7.02 amu Calculate the atomic mass of lithium (Li) and express answer to 4 significant figures with the appropriate unit.
Both lithium (Li) and silver (Ag) have 2 naturally occurring isotopes. Calculate the atomic mass of each element using the given data for the % relative abundance and mass of each isotope. A) 1st isotope: Li-6 has % relative abundance = 7.42% and mass = 6.01 amu 2nd isotope: Li-7 has % relative abundance = 92.58% and mass = 7.02 amu Calculate the atomic mass of lithium (Li) and express answer to 4 significant figures with the appropriate unit.
General, Organic, and Biological Chemistry
7th Edition
ISBN:9781285853918
Author:H. Stephen Stoker
Publisher:H. Stephen Stoker
Chapter3: Atomic Structures And The Periodic Table
Section: Chapter Questions
Problem 3.32EP: Calculate the atomic mass of each of the following elements using the given data for the percentage...
Related questions
Question
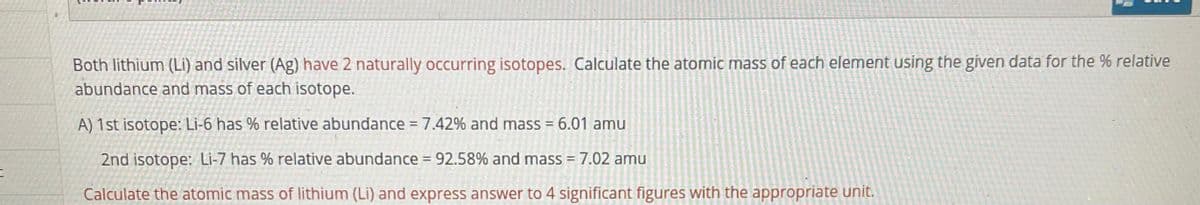
Transcribed Image Text:Both lithium (Li) and silver (Ag) have 2 naturally occurring isotopes. Calculate the atomic mass of each element using the given data for the % relative
abundance and mass of each isotope.
A) 1st isotope: Li-6 has % relative abundance =7.42% and mass = 6.01 amu
2nd isotope: Li-7 has % relative abundance = 92.58% and mass = 7.02 amu
Calculate the atomic mass of lithium (Li) and express answer to 4 significant figures with the appropriate unit.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Recommended textbooks for you

General, Organic, and Biological Chemistry
Chemistry
ISBN:
9781285853918
Author:
H. Stephen Stoker
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning


General, Organic, and Biological Chemistry
Chemistry
ISBN:
9781285853918
Author:
H. Stephen Stoker
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning


Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning