Bottom Top Water temperature, °F Water vapor pressure, psia 120 126 1.69 1.995 Mole fraction H,O in air 0.001609 0.0882 Total pressure, psia 14.1 14.3 Air rate, Ibmol/h 0.401 0.401 Column cross-sectional area, fr 0.5 0.5 Water rate, Ibmol/h (approximation) 20 20
Bottom Top Water temperature, °F Water vapor pressure, psia 120 126 1.69 1.995 Mole fraction H,O in air 0.001609 0.0882 Total pressure, psia 14.1 14.3 Air rate, Ibmol/h 0.401 0.401 Column cross-sectional area, fr 0.5 0.5 Water rate, Ibmol/h (approximation) 20 20
Introduction to Chemical Engineering Thermodynamics
8th Edition
ISBN:9781259696527
Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Chapter1: Introduction
Section: Chapter Questions
Problem 1.1P
Related questions
Question
Overall mass-transfer coefficient for a packed cooling tower.
A cooling-tower packing was tested in a small column. At two points in the column, 0.7 ft apart, the data below apply. Calculate the overall volumetric mass-transfer coefficient Kya that can be used to design a large, packed-bed cooling tower, where a is the masstransfer area, A, per unit volume, V, of tower.
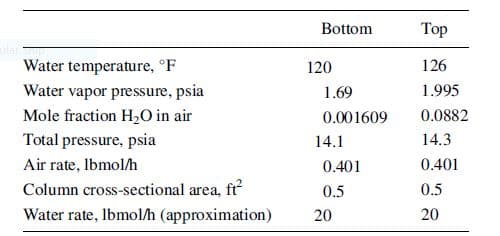
Transcribed Image Text:Bottom
Top
Water temperature, °F
Water vapor pressure, psia
120
126
1.69
1.995
Mole fraction H,O in air
0.001609
0.0882
Total pressure, psia
14.1
14.3
Air rate, Ibmol/h
0.401
0.401
Column cross-sectional area, fr
0.5
0.5
Water rate, Ibmol/h (approximation)
20
20
Expert Solution
Trending now
This is a popular solution!
Step by step
Solved in 9 steps with 9 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemical-engineering and related others by exploring similar questions and additional content below.Recommended textbooks for you

Introduction to Chemical Engineering Thermodynami…
Chemical Engineering
ISBN:
9781259696527
Author:
J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher:
McGraw-Hill Education

Elementary Principles of Chemical Processes, Bind…
Chemical Engineering
ISBN:
9781118431221
Author:
Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:
WILEY

Elements of Chemical Reaction Engineering (5th Ed…
Chemical Engineering
ISBN:
9780133887518
Author:
H. Scott Fogler
Publisher:
Prentice Hall

Introduction to Chemical Engineering Thermodynami…
Chemical Engineering
ISBN:
9781259696527
Author:
J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher:
McGraw-Hill Education

Elementary Principles of Chemical Processes, Bind…
Chemical Engineering
ISBN:
9781118431221
Author:
Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:
WILEY

Elements of Chemical Reaction Engineering (5th Ed…
Chemical Engineering
ISBN:
9780133887518
Author:
H. Scott Fogler
Publisher:
Prentice Hall


Industrial Plastics: Theory and Applications
Chemical Engineering
ISBN:
9781285061238
Author:
Lokensgard, Erik
Publisher:
Delmar Cengage Learning

Unit Operations of Chemical Engineering
Chemical Engineering
ISBN:
9780072848236
Author:
Warren McCabe, Julian C. Smith, Peter Harriott
Publisher:
McGraw-Hill Companies, The