BOYLE's LAW A balloon, originally at 2.21 iters and 1.01 atmospheres is expanded to 23.4 liters. What is the balloon's new pressure? A balloon, originally at 2.21 iters and 1.01 atmospheres, has its pressure decreased to 0.0211 atmospheres. What is the balloon's new volume?
BOYLE's LAW A balloon, originally at 2.21 iters and 1.01 atmospheres is expanded to 23.4 liters. What is the balloon's new pressure? A balloon, originally at 2.21 iters and 1.01 atmospheres, has its pressure decreased to 0.0211 atmospheres. What is the balloon's new volume?
Introductory Chemistry: A Foundation
9th Edition
ISBN:9781337399425
Author:Steven S. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Donald J. DeCoste
Chapter13: Gases
Section: Chapter Questions
Problem 114AP
Related questions
Question

Transcribed Image Text:ormal text
Arial
18
BIUA
Practice Questions Using the Gas Laws
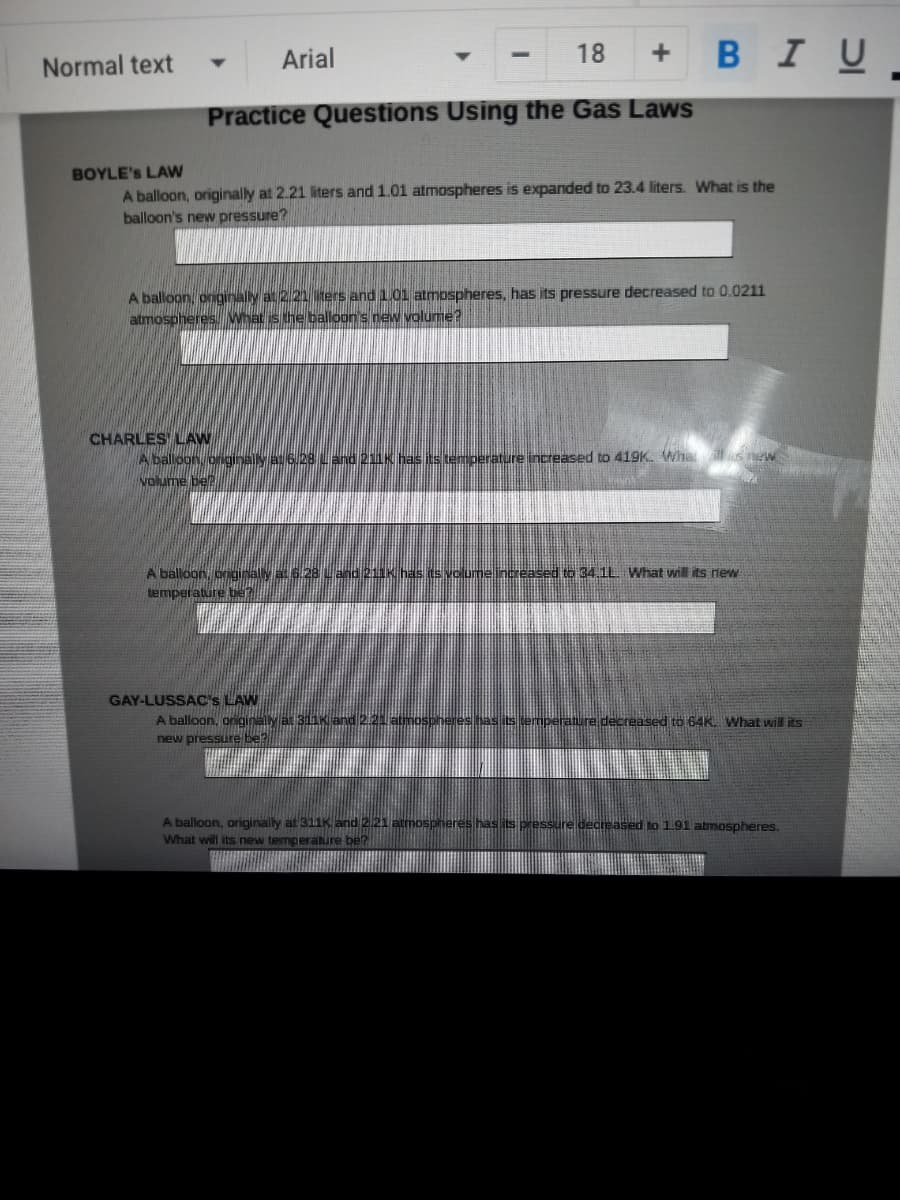
BOYLE's LAW
A balloon, originally at 2.21 iters and 1.01 atmospheres is expanded to 23.4 liters. Wwhat is the
balloon's new pressure?
A balloon, originally at 2.21 liters and 1.01 atmospheres, has its pressure decreased to 0.0211
atmospheres. What is the balloon's new volume?
CHARLES' LAW
A balloon, originally at 6.28 L and 211K has its temperature increased to 410K Whatits
volume be?
A balloon, originally at 6.28 L and 211K has its volume increased to 34.1L. What will its new
Nemperature be?
GAY-LUSSAC's LAW
A balloon, originally at 311K and 2.21 atmospheres has its bemperature decreased to 64K. What will its
new pressure be?
A balloon, originally at 311K and 2.21 atmospheres has its pressure decreased to 191 atmospheres.
What will its new temperature be?
Transcribed Image Text:в I U
Normal text
Arial
18
Practice Questions Using the Gas Laws
BOYLE's LAW
A balloon, originally at 2.21 liters and 1.01 atmospheres is expanded to 23.4 liters. What is the
balloon's new pressure?
A balloon, onginally at 2.21 ters and 1.01 atmospheres, has its pressure decreased to 0.0211
atmospheres What is the balloon's new volume?
CHARLES LAW
A balloon originalMa 6U28 Land 21IK Gas is teT perature increased to 419K. Wha us new
volume be?
A balloon, onginaly at 6.28 Land 211K has its volumeincreased to 34.1L. What will its new
temperature be?
GAY-LUSSAC's LAW
A balloon, griginally at BI
temperature decreased to 64K. What will its
new pressure be
A balloon, originally at 311K and 2.21 atmospheres
What will its new temperature be?
decreased to 191 atmospheres.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

General, Organic, and Biological Chemistry
Chemistry
ISBN:
9781285853918
Author:
H. Stephen Stoker
Publisher:
Cengage Learning