Question Choices A AH corresponds to an Negative, exothermic Negative, endothermic process. Positive, exothermic Zero, endothermic Zero, exothermic The internal energy can be increased by I. transferring heat from the surroundings to the system II. transferring heat from the system to the surroundings III. doing work on the system III only Il only Il and III I and II The value of AH° for the reaction 0.182 CH,OH, → CO) + 2H20) is +128.1 kJ. Determine the heat consumed when 8.31 23.3 5.10 g of CO) is formed. 162
Question Choices A AH corresponds to an Negative, exothermic Negative, endothermic process. Positive, exothermic Zero, endothermic Zero, exothermic The internal energy can be increased by I. transferring heat from the surroundings to the system II. transferring heat from the system to the surroundings III. doing work on the system III only Il only Il and III I and II The value of AH° for the reaction 0.182 CH,OH, → CO) + 2H20) is +128.1 kJ. Determine the heat consumed when 8.31 23.3 5.10 g of CO) is formed. 162
Principles of Modern Chemistry
8th Edition
ISBN:9781305079113
Author:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Chapter12: Thermodynamic Processes And Thermochemistry
Section: Chapter Questions
Problem 10P
Related questions
Question
1. COMPLETE THE TABLE
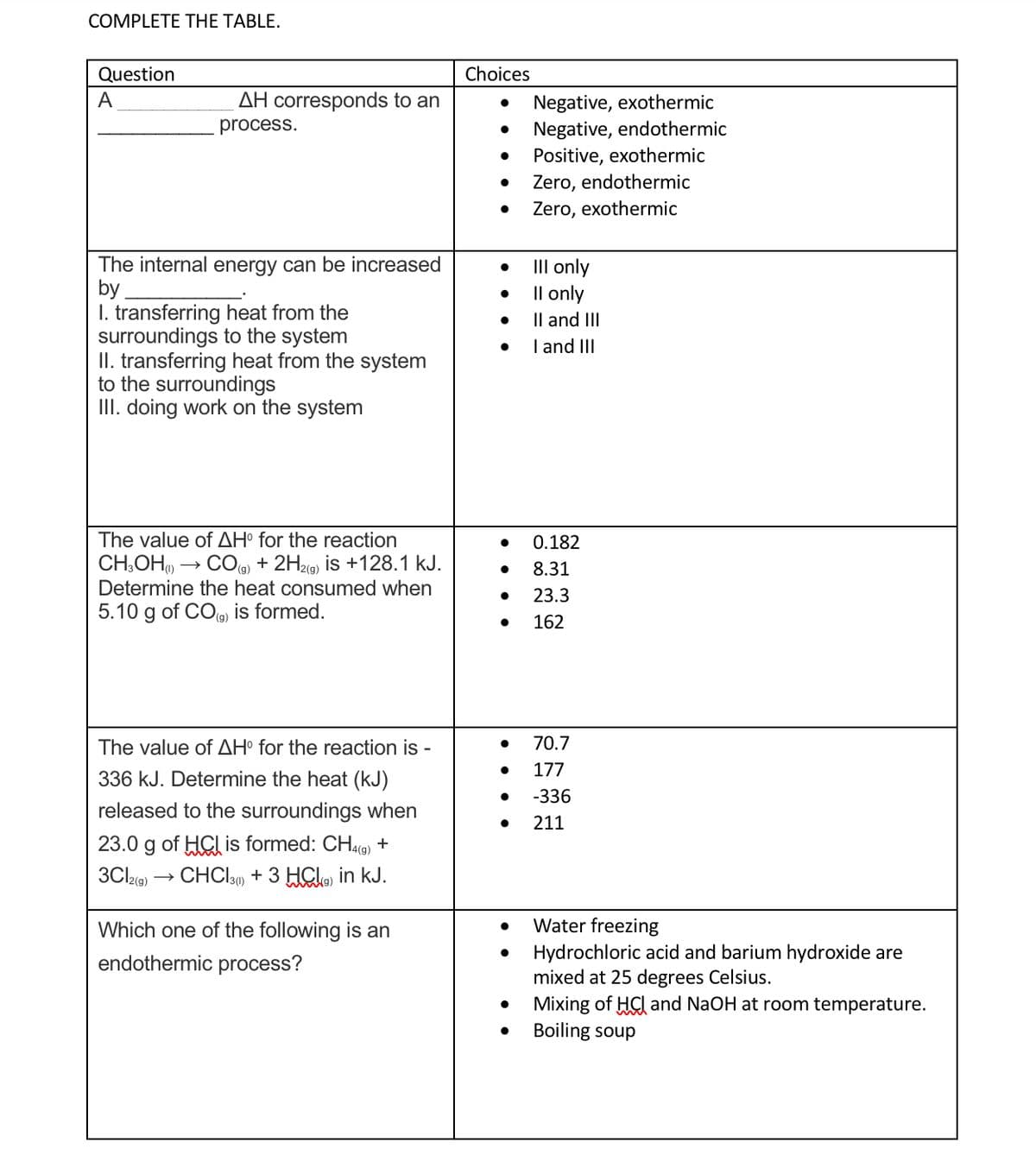
Transcribed Image Text:COMPLETE THE TABLE.
Question
Choices
A
AH corresponds to an
Negative, exothermic
Negative, endothermic
Positive, exothermic
process.
Zero, endothermic
Zero, exothermic
The internal energy can be increased
by
I. transferring heat from the
surroundings to the system
II. transferring heat from the system
to the surroundings
III. doing work on the system
III only
Il only
Il and III
I and III
The value of AH° for the reaction
0.182
CH,OH) → CO + 2H2(9) is +128.1 kJ.
Determine the heat consumed when
8.31
23.3
5.10 g of COe) is formed.
162
The value of AH° for the reaction is -
70.7
177
336 kJ. Determine the heat (kJ)
-336
released to the surroundings when
211
23.0 g of HGl is formed: CHa9) +
3CI2G) → CHCIu) + 3 HClo in kJ.
Which one of the following is an
Water freezing
• Hydrochloric acid and barium hydroxide are
mixed at 25 degrees Celsius.
Mixing of HCI and NaOH at room temperature.
Boiling soup
endothermic process?
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning