By what factor does increasing the temperature of a reaction from T₁ = 289 K to T₂ = 299 K increase the rate of reaction? Assume the activation energy (Ea) of this reaction is 164,500 J and that the pre-exponential constant (A) is 2.1 x 10⁹ s-¹. Express your answer to one decimal place. k₂/k1 = 17| ΑΣΦ ?
By what factor does increasing the temperature of a reaction from T₁ = 289 K to T₂ = 299 K increase the rate of reaction? Assume the activation energy (Ea) of this reaction is 164,500 J and that the pre-exponential constant (A) is 2.1 x 10⁹ s-¹. Express your answer to one decimal place. k₂/k1 = 17| ΑΣΦ ?
Chemistry: The Molecular Science
5th Edition
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:John W. Moore, Conrad L. Stanitski
Chapter11: Chemical Kinetics: Rates Of Reactions
Section: Chapter Questions
Problem 11.ACP: (Section 11-5) A rule of thumb is that for a typical reaction, if concentrations are unchanged, a...
Related questions
Question
8L.13.1
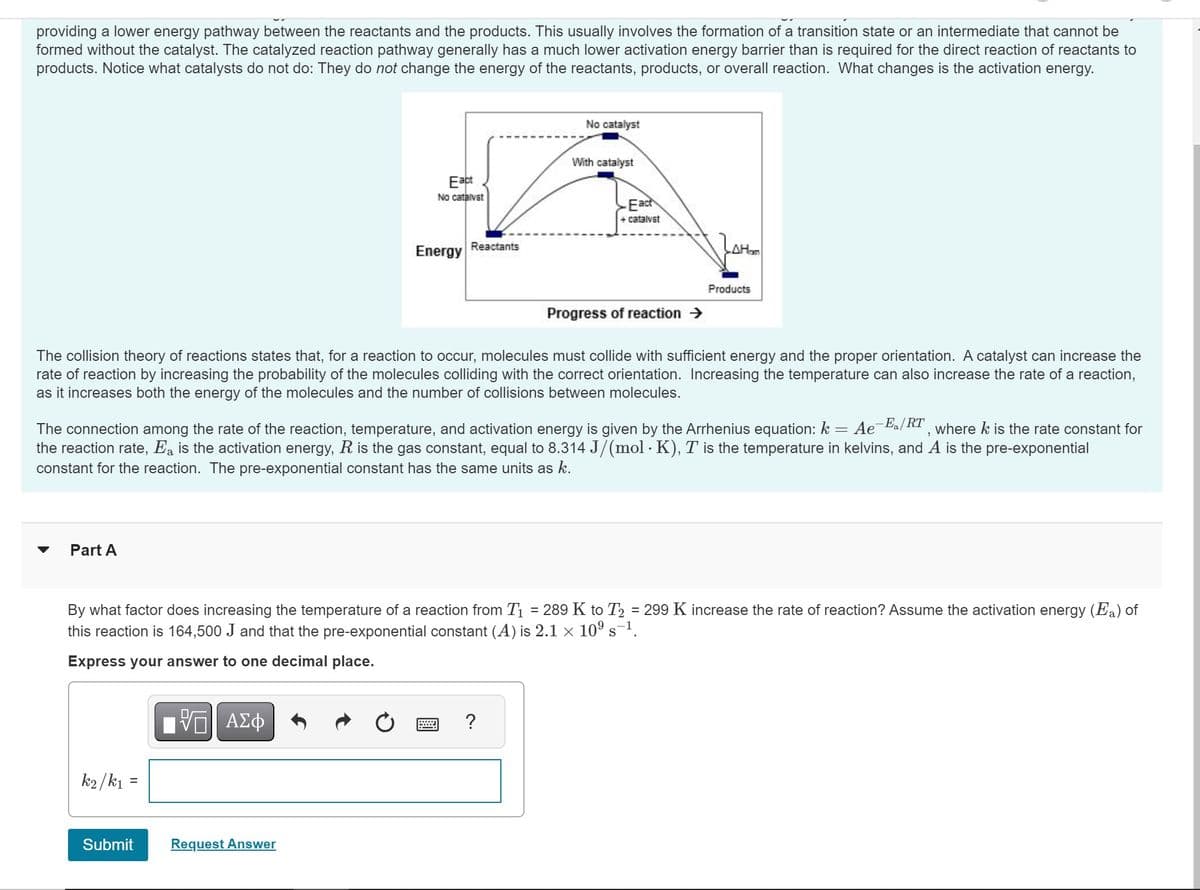
Transcribed Image Text:providing a lower energy pathway between the reactants and the products. This usually involves the formation of a transition state or an intermediate that cannot be
formed without the catalyst. The catalyzed reaction pathway generally has a much lower activation energy barrier than is required for the direct reaction of reactants to
products. Notice what catalysts do not do: They do not change the energy of the reactants, products, or overall reaction. What changes is the activation energy.
Part A
k₂/k1
=
Eact
No catalvat
Submit
Energy Reactants
——| ΑΣΦ
The collision theory of reactions states that, for a reaction to occur, molecules must collide with sufficient energy and the proper orientation. A catalyst can increase the
rate of reaction by increasing the probability of the molecules colliding with the correct orientation. Increasing the temperature can also increase the rate of a reaction,
as it increases both the energy of the molecules and the number of collisions between molecules.
Request Answer
No catalyst
=
The connection among the rate of the reaction, temperature, and activation energy is given by the Arrhenius equation: k Ae-Ea/RT, where k is the rate constant for
the reaction rate, Ea is the activation energy, R is the gas constant, equal to 8.314 J/(mol · K), T is the temperature in kelvins, and A is the pre-exponential
constant for the reaction. The pre-exponential constant has the same units as k.
With catalyst
Eact
+ catalyst
By what factor does increasing the temperature of a reaction from T₁ = 289 K to T₂ = 299 K increase the rate of reaction? Assume the activation energy (Ea) of
this reaction is 164,500 J and that the pre-exponential constant (A) is 2.1 × 10⁰⁹ s-¹.
Express your answer to one decimal place.
?
Progress of reaction →
LAH₂
Products
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 3 steps with 6 images

Follow-up Questions
Read through expert solutions to related follow-up questions below.
Follow-up Question
8L.13.3

Transcribed Image Text:The rate of an enzyme-catalyzed reaction is 2.26×105 times faster than the rate of the uncatalyzed reaction. What is the difference in Ea between the uncatalyzed
and catalyzed reactions at T = 262K ?
Express your answer with the appropriate units.
μA
Value
Units
?
Solution
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning


Chemistry for Today: General, Organic, and Bioche…
Chemistry
ISBN:
9781305960060
Author:
Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:
Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning