c) Answer ALL parts (i) to (v)- (!) (i) Draw the structure of th of the ester 4 by the bas (ii) Suggest a mechanism f its reaction with ethanc (iii) For each diastereoisom conformations (4 chair (iv) By referring to your ch diastereoisomer (4 or 6 been reached. (v) What do you think the performed using NaOM NaOEt O H OEt HO. Me 4.
c) Answer ALL parts (i) to (v)- (!) (i) Draw the structure of th of the ester 4 by the bas (ii) Suggest a mechanism f its reaction with ethanc (iii) For each diastereoisom conformations (4 chair (iv) By referring to your ch diastereoisomer (4 or 6 been reached. (v) What do you think the performed using NaOM NaOEt O H OEt HO. Me 4.
Organic Chemistry
8th Edition
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Chapter23: Amines
Section: Chapter Questions
Problem 23.37P
Related questions
Question
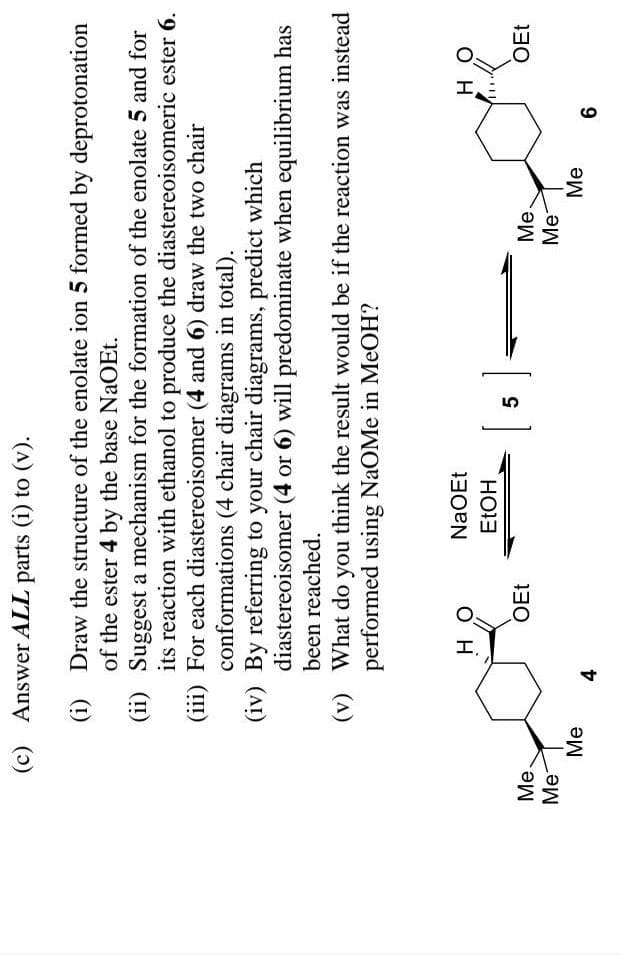
Transcribed Image Text:(c) Answer ALL parts (i) to (v).
(i) Draw the structure of the enolate ion 5 formed by deprotonation
of the ester 4 by the base NaOEt.
(ii) Suggest a mechanism for the formation of the enolate 5 and for
its reaction with ethanol to produce the diastereoisomeric ester 6.
(iii) For each diastereoisomer (4 and 6) draw the two chair
conformations (4 chair diagrams in total).
(iv) By referring to your chair diagrams, predict which
diastereoisomer (4 or 6) will predominate when equilibrium has
been reached.
(v) What do you think the result would be if the reaction was instead
performed using NaOMe in MeOH?
NaOEt
O H
он
OEt
OEt
Ме.
Me
Ме
Ме.
Me-
Me
4.
9
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning

Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning