c) What is the pH of the groundwater sample in which dichromate is óxidi2e ethanol, which is simultaneously transformed to dissolved carbon dioxide? Modeling studies show that equilibrium is 1.75 x 107 M, dichromate ion is 7.3 x 102 M, chromic ion is 8.2 x 10°, ethanol is 6.8 x 102, and carbon dioxide is 6.4 x 108. di for the state of South Carolina, describe
c) What is the pH of the groundwater sample in which dichromate is óxidi2e ethanol, which is simultaneously transformed to dissolved carbon dioxide? Modeling studies show that equilibrium is 1.75 x 107 M, dichromate ion is 7.3 x 102 M, chromic ion is 8.2 x 10°, ethanol is 6.8 x 102, and carbon dioxide is 6.4 x 108. di for the state of South Carolina, describe
Chemistry for Engineering Students
4th Edition
ISBN:9781337398909
Author:Lawrence S. Brown, Tom Holme
Publisher:Lawrence S. Brown, Tom Holme
Chapter12: Chemical Equilibrium
Section: Chapter Questions
Problem 12.101PAE: 12.101 An engineer working on a design to extract petroleum from a deep thermal reservoir wishes to...
Related questions
Question
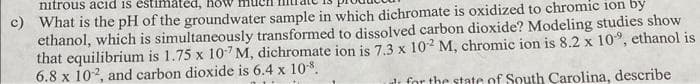
Transcribed Image Text:nitrous acid is estimated,
c) What is the pH of the groundwater sample in which dichromate is oxidized to chromic ion by
ethanol, which is simultaneously transformed to dissolved carbon dioxide? Modeling studies show
that equilibrium is 1.75 x 107 M, dichromate ion is 7.3 x 10-2 M, chromic ion is 8.2 x 10°, ethanol is
6.8 x 102, and carbon dioxide is 6.4 x 10%.
dr for the state of South Carolina, describe
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 3 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning

World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning