Prepare a standard curve from the data below from a Bradford assay. Samples were prepared from a 0.100 mg/mL egg white lysozyme stock solution in a 100.0 mM, pH 7.0 phosphate buffer at various dilutions in a final volume of 1.0 mL. The data is shown in the table below: Dilution (%) 0.0 (blank) 2.5 → 0.0025 mg 5.0 → 0.005 mg Absorbance @ 595 nm (1 cm pathlength) 0.000 0.031 0.075 10.0 → 0.01 mg 0.134 15.0 → 0.015 0.229 20.0 → 0.02 0.280 25.0 → 0.025 0.386 37.5 → 0.0375 0.540 50.0 → 0.05 mg 0.742
Prepare a standard curve from the data below from a Bradford assay. Samples were prepared from a 0.100 mg/mL egg white lysozyme stock solution in a 100.0 mM, pH 7.0 phosphate buffer at various dilutions in a final volume of 1.0 mL. The data is shown in the table below: Dilution (%) 0.0 (blank) 2.5 → 0.0025 mg 5.0 → 0.005 mg Absorbance @ 595 nm (1 cm pathlength) 0.000 0.031 0.075 10.0 → 0.01 mg 0.134 15.0 → 0.015 0.229 20.0 → 0.02 0.280 25.0 → 0.025 0.386 37.5 → 0.0375 0.540 50.0 → 0.05 mg 0.742
Chapter14: Chromatography
Section: Chapter Questions
Problem 1P
Related questions
Question
Plot a professional quality standard curve (absorbance vs. protein amount in µg) with an appropriate title and caption underneath the plot from the data give above. The caption should include the best-fit data.
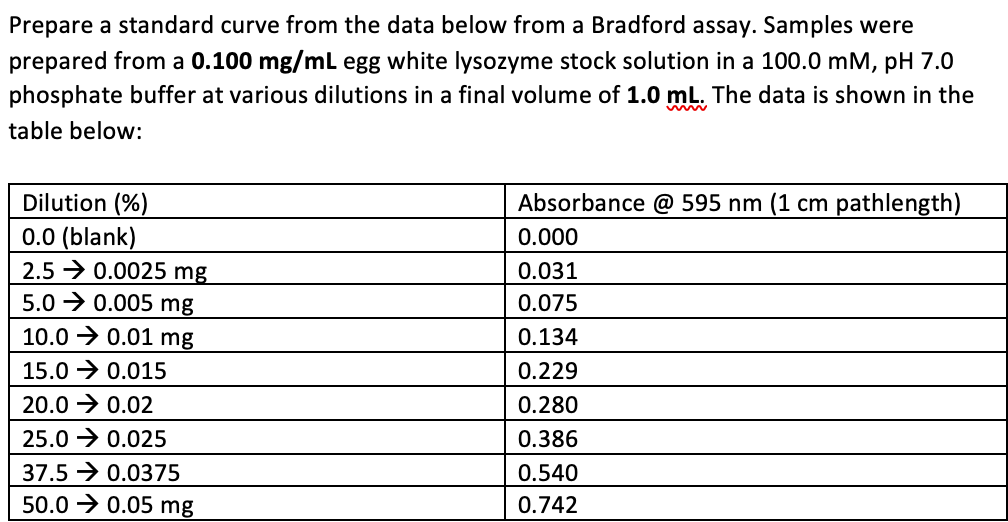
Transcribed Image Text:Prepare a standard curve from the data below from a Bradford assay. Samples were
prepared from a 0.100 mg/mL egg white lysozyme stock solution in a 100.0 mM, pH 7.0
phosphate buffer at various dilutions in a final volume of 1.0 mL. The data is shown in the
table below:
Dilution (%)
Absorbance @ 595 nm (1 cm pathlength)
0.0 (blank)
2.5 → 0.0025 mg
5.0 → 0.005 mg
0.000
0.031
0.075
10.0 → 0.01 mg
0.134
15.0 → 0.015
0.229
20.0 → 0.02
0.280
25.0 → 0.025
0.386
37.5 → 0.0375
0.540
50.0 → 0.05 mg
0.742
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you


Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning



Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning
