c. A landmark study into the mechanism of acetal chemistry started with radiolabeled benzaldehyde, wherein the oxygen of the aldehyde was replaced with its heavier isotope oxygen-18 (1®O). The radiolabeled aldehyde was treated with non-radiolabeled n-butyl alcohol in the presence of strong acid catalyst as shown in the complete reaction equation shown below, without radiolabeling in the products. Based on your knowledge of the reaction mechanism, circle any oxygen atom(s) in the products shown that should be radiolabeled, and explain your logic in one sentence. Но. `H + H20 H* cat.
c. A landmark study into the mechanism of acetal chemistry started with radiolabeled benzaldehyde, wherein the oxygen of the aldehyde was replaced with its heavier isotope oxygen-18 (1®O). The radiolabeled aldehyde was treated with non-radiolabeled n-butyl alcohol in the presence of strong acid catalyst as shown in the complete reaction equation shown below, without radiolabeling in the products. Based on your knowledge of the reaction mechanism, circle any oxygen atom(s) in the products shown that should be radiolabeled, and explain your logic in one sentence. Но. `H + H20 H* cat.
Organic Chemistry
8th Edition
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Chapter24: Catalytic Carbon-carbon Bond Formation
Section: Chapter Questions
Problem 24.36P
Related questions
Question
please answer part c
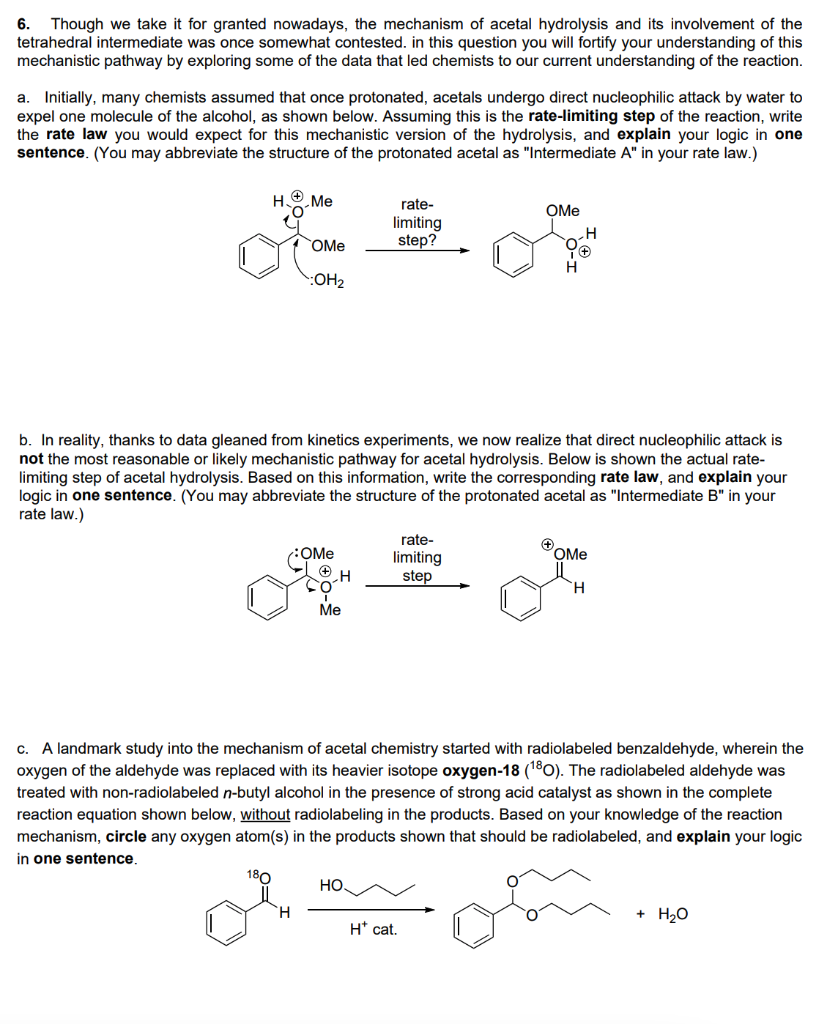
Transcribed Image Text:6. Though we take it for granted nowadays, the mechanism of acetal hydrolysis and its involvement of the
tetrahedral intermediate was once somewhat contested. in this question you will fortify your understanding of this
mechanistic pathway by exploring some of the data that led chemists to our current understanding of the reaction.
a. Initially, many chemists assumed that once protonated, acetals undergo direct nucleophilic attack by water to
expel one molecule of the alcohol, as shown below. Assuming this is the rate-limiting step of the reaction, write
the rate law you would expect for this mechanistic version of the hydrolysis, and explain your logic in one
sentence. (You may abbreviate the structure of the protonated acetal as "Intermediate A" in your rate law.)
H
Me
rate-
OMe
limiting
step?
OMe
:OH2
b. In reality, thanks to data gleaned from kinetics experiments, we now realize that direct nucleophilic attack is
not the most reasonable or likely mechanistic pathway for acetal hydrolysis. Below is shown the actual rate-
limiting step of acetal hydrolysis. Based on this information, write the corresponding rate law, and explain your
logic in one sentence. (You may abbreviate the structure of the protonated acetal as "Intermediate B" in your
rate law.)
rate-
limiting
step
:OMe
OMe
H.
Ме
c. A landmark study into the mechanism of acetal chemistry started with radiolabeled benzaldehyde, wherein the
oxygen of the aldehyde was replaced with its heavier isotope oxygen-18 (180). The radiolabeled aldehyde was
treated with non-radiolabeled n-butyl alcohol in the presence of strong acid catalyst as shown in the complete
reaction equation shown below, without radiolabeling in the products. Based on your knowledge of the reaction
mechanism, circle any oxygen atom(s) in the products shown that should be radiolabeled, and explain your logic
in one sentence,
180
НО.
+ H20
H* cat.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning

Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning