C. Thermite welding is an exothermic reaction, which produces molten iron that permanently binds metals as it cools. The process does not require heat or electricity. Aluminum powder is simply mixed with ferric oxide powder. The reaction is accompanied by a release of heat, and produces aluminum oxide and molten iron. Write the balanced equation for the reaction. If 55.0 grams of aluminum powder is mixed with 75.0 grams of ferric oxide, which of the two substances is the limiting reagent? the excess reagent? How many grams of molten iron will be produced?
C. Thermite welding is an exothermic reaction, which produces molten iron that permanently binds metals as it cools. The process does not require heat or electricity. Aluminum powder is simply mixed with ferric oxide powder. The reaction is accompanied by a release of heat, and produces aluminum oxide and molten iron. Write the balanced equation for the reaction. If 55.0 grams of aluminum powder is mixed with 75.0 grams of ferric oxide, which of the two substances is the limiting reagent? the excess reagent? How many grams of molten iron will be produced?
Chemistry: Matter and Change
1st Edition
ISBN:9780078746376
Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Chapter11: Stoichiometry
Section: Chapter Questions
Problem 70A
Related questions
Question
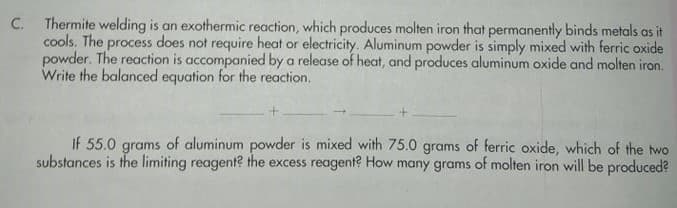
Transcribed Image Text:C. Thermite welding is an exothermic reaction, which produces molten iron that permanently binds metals as it
cools. The process does not require heat or electricity. Aluminum powder is simply mixed with ferric oxide
powder. The reaction is accompanied by a release of heat, and produces aluminum oxide and molten iron.
Write the balanced equation for the reaction.
If 55.0 grams of aluminum powder is mixed with 75.0 grams of ferric oxide, which of the two
substances is the limiting reagent? the excess reagent? How many grams of molten iron will be produced?
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: Matter and Change
Chemistry
ISBN:
9780078746376
Author:
Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:
Glencoe/McGraw-Hill School Pub Co

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:
9780078746376
Author:
Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:
Glencoe/McGraw-Hill School Pub Co

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning
