c. Using the results from part (a), how long will it take for the paint to completely dry? d. What are the hydrodynamic (8) and concentration boundary- layer (6) thicknesses at x=L=1.5 m? How does this compare to H, the height of the drying chamber? What is the new required air flow rate (m³/min) if the desired solvent mass-transfer rate is 150 g/min?
c. Using the results from part (a), how long will it take for the paint to completely dry? d. What are the hydrodynamic (8) and concentration boundary- layer (6) thicknesses at x=L=1.5 m? How does this compare to H, the height of the drying chamber? What is the new required air flow rate (m³/min) if the desired solvent mass-transfer rate is 150 g/min?
Principles of Heat Transfer (Activate Learning with these NEW titles from Engineering!)
8th Edition
ISBN:9781305387102
Author:Kreith, Frank; Manglik, Raj M.
Publisher:Kreith, Frank; Manglik, Raj M.
Chapter9: Heat Transfer With Phase Change
Section: Chapter Questions
Problem 9.28P
Related questions
Question
Please state when values are sources from an appendix/table.

Transcribed Image Text:Using the results from part (a), how long will it take for the
paint to completely dry?
d. What are the hydrodynamic (8) and concentration boundary-
layer (8) thicknesses at x=L=1.5 m? How does this
compare to H, the height of the drying chamber?
What is the new required air flow rate (m³/min) if the desired
solvent mass-transfer rate is 150 g/min?
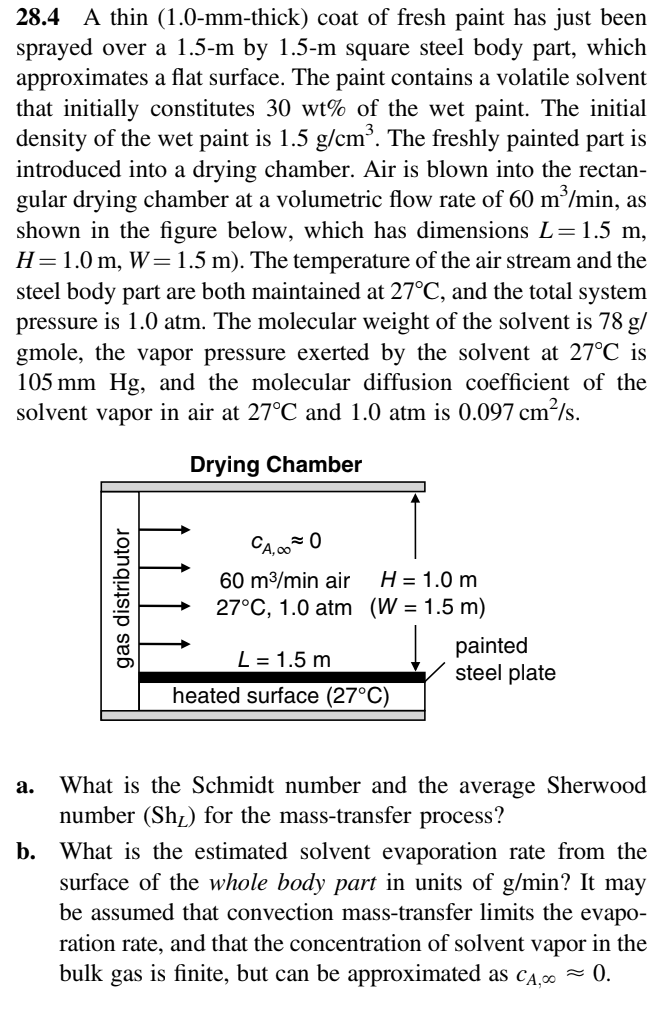
Transcribed Image Text:28.4 A thin (1.0-mm-thick) coat of fresh paint has just been
sprayed over a 1.5-m by 1.5-m square steel body part, which
approximates a flat surface. The paint contains a volatile solvent
that initially constitutes 30 wt% of the wet paint. The initial
density of the wet paint is 1.5 g/cm³. The freshly painted part is
introduced into a drying chamber. Air is blown into the rectan-
gular drying chamber at a volumetric flow rate of 60 m³/min, as
shown in the figure below, which has dimensions L = 1.5 m,
H=1.0 m, W= 1.5 m). The temperature of the air stream and the
steel body part are both maintained at 27°C, and the total system
pressure is 1.0 atm. The molecular weight of the solvent is 78 g/
gmole, the vapor pressure exerted by the solvent at 27°C is
105 mm Hg, and the molecular diffusion coefficient of the
solvent vapor in air at 27°C and 1.0 atm is 0.097 cm²/s.
Drying Chamber
gas distributor
CA,000
60 m³/min air
H = 1.0 m
27°C, 1.0 atm (W = 1.5 m)
L = 1.5 m
heated surface (27°C)
painted
steel plate
a. What is the Schmidt number and the average Sherwood
number (Sh₂) for the mass-transfer process?
b. What is the estimated solvent evaporation rate from the
surface of the whole body part in units of g/min? It may
be assumed that convection mass-transfer limits the evapo-
ration rate, and that the concentration of solvent vapor in the
bulk gas is finite, but can be approximated as CA, ≈ 0.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 9 steps with 8 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, mechanical-engineering and related others by exploring similar questions and additional content below.Recommended textbooks for you

Principles of Heat Transfer (Activate Learning wi…
Mechanical Engineering
ISBN:
9781305387102
Author:
Kreith, Frank; Manglik, Raj M.
Publisher:
Cengage Learning

Principles of Heat Transfer (Activate Learning wi…
Mechanical Engineering
ISBN:
9781305387102
Author:
Kreith, Frank; Manglik, Raj M.
Publisher:
Cengage Learning