c. When 9.5 g of an impure Silver Nitrate (AGNO, mol. wt 170 g/mol) was treated with excess sodium chloride (NaCl), 7.15 g of a precipitate (mol wt. 143 g/mol) was obtained. How many grams of AgNO3 was in the mixture? Only number needed Move To...
c. When 9.5 g of an impure Silver Nitrate (AGNO, mol. wt 170 g/mol) was treated with excess sodium chloride (NaCl), 7.15 g of a precipitate (mol wt. 143 g/mol) was obtained. How many grams of AgNO3 was in the mixture? Only number needed Move To...
Chemistry by OpenStax (2015-05-04)
1st Edition
ISBN:9781938168390
Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Chapter3: Composition Of Substances And Solutions
Section: Chapter Questions
Problem 64E: What is the final concentration of the solution produced when 225.5 mL of a 0.09988-M solution of...
Related questions
Question
100%
Please help with these!

Transcribed Image Text:c. When 9.5 g of an impure Silver Nitrate (AGNO3, mol. wt 170 g/mol) was treated with excess
sodium chloride (NaCI), 7.15 g of a precipitate (mol wt. 143 g/mol) was obtained. How many
grams of AgNO, was in the mixture? Only number needed
Move To...
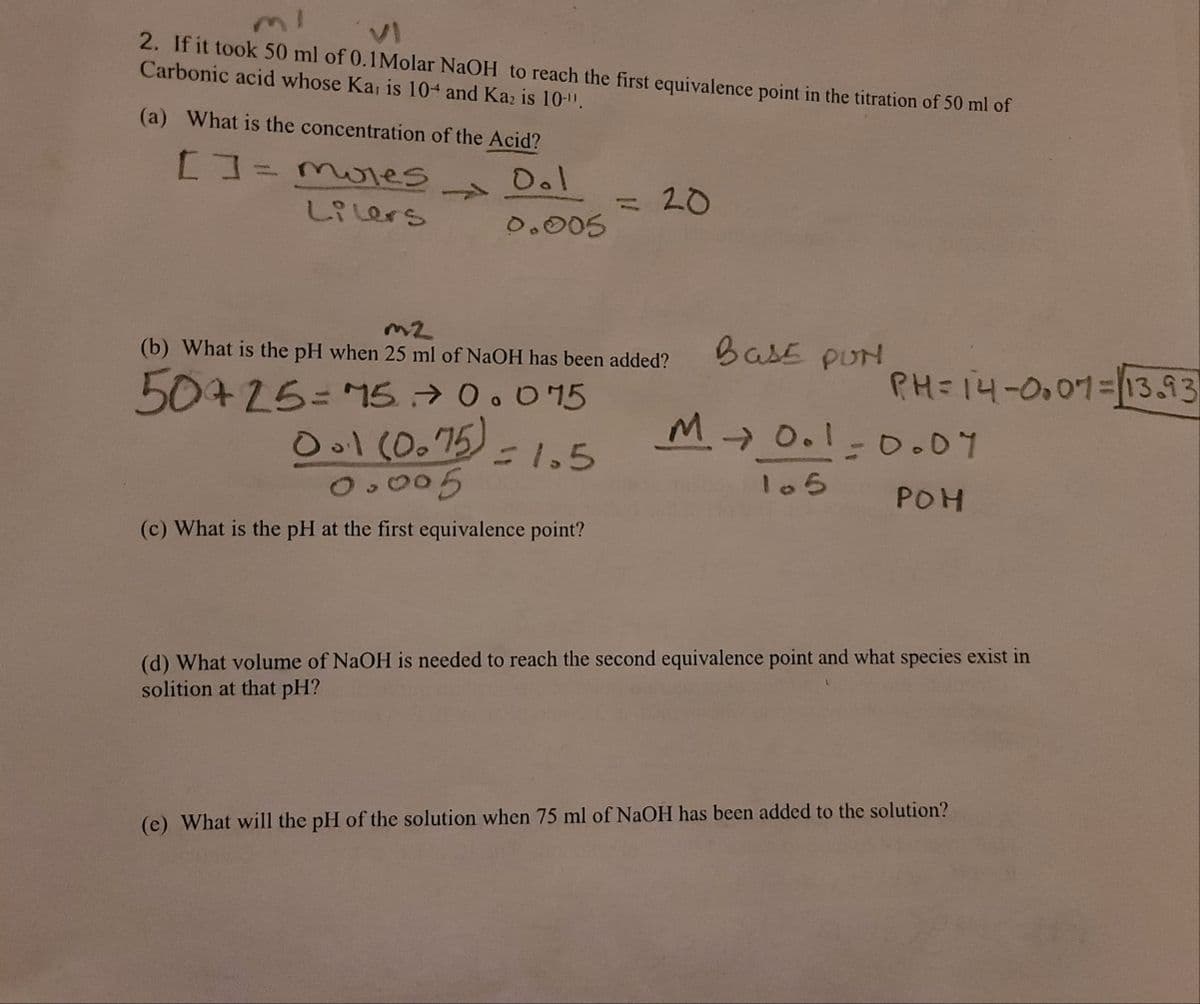
Transcribed Image Text:2. If it took 50 ml of 0.1Molar NaOH to reach the first equivalence point in the titration of 50 ml of
Carbonic acid whose Ka, is 10+ and Kaz is 10-".
(a) What is the concentration of the Acid?
D.l
1.
20
%3D
しR Lers
0.005
BasE pUN
(b) What is the pH when 25 ml of NaOH has been added?
PH=14-0.01=13.93
50+25= 15 → 0.0 15
Ool (0.75) = 1.5
0.005
M→ 0.1-0.07
105
POH
(c) What is the pH at the first equivalence point?
(d) What volume of NaOH is needed to reach the second equivalence point and what species exist in
solition at that pH?
(c) What will the pH of the solution when 75 ml of NaOH has been added to the solution?
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning



Chemistry: Matter and Change
Chemistry
ISBN:
9780078746376
Author:
Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:
Glencoe/McGraw-Hill School Pub Co

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning