Calculate AG for the following reaction. 2 Cr(s) +3 Cl2(aq) → 2 Cr** (aq) + 6 CI (aq) Hall-Reaction Hall-Reaction 2.87 1.99 0,+ 2H,0 + → LOH" C+ 2Cu Hg C, + 2 2Hg + 20 Aa +eA +a so+ AH + 2I,S0, + HO Ag+e Ag Co +eCo 1. 0.40 0.34 1.82 1.78 1,70 0.27 0.22 0.30 Ce+e Ce PO - 4H + SO + 2PASO, + 2H,0 MaO,+4H + 3Ma0, + ZH,0 2+ 2H + 10 →10," + HO Ma0,+ KH" + SeMa"+ 4H0 Au +3Au PhO, + 4H + 2e + 2H,0 016 168 2H + 2eB 0.00 -0.06 -Q13 160 131 P+2P Sa +2e Sa N4 2e- NI PASO, + P+ so, 10 -014 146 1.36 1.33 1,23 1.21 -035 Cr0, + 14H" + e2C" + 7HO 0,+4H + → 2H,0 MaOy + 4H +2e Ma + 2H,0 10, + GH + S4 + 3H,0 Bey+ 32Br VO," + 2H +e VO+ H0 AC+ 3 Au + 4a NO, +4H +3eNO + 21,0 -040 -041 -044 -G73 --ax 1.20 Z + 2- Za 2H,0 + 2e-H, + 201 Ma+ 2e Ma A+ Al H+ 2e2H Mg+2eMg La+ La Na" +e-Na C+ 2eCa 1.09 1.00 -LII 0.99 0.96 -166 -223 -237 0.954 0.91 2Hg + 2eHg" Ag +eAg H+2e"2Hg -2.37 -2.71 ON0 -276 -2.90 0.77 0.58 0, + 2H" + 2e -HO, MaO, Mo0, 6+ 2e" 21 -292 -3.05 K* +eK 0.56 0.4 0.52
Calculate AG for the following reaction. 2 Cr(s) +3 Cl2(aq) → 2 Cr** (aq) + 6 CI (aq) Hall-Reaction Hall-Reaction 2.87 1.99 0,+ 2H,0 + → LOH" C+ 2Cu Hg C, + 2 2Hg + 20 Aa +eA +a so+ AH + 2I,S0, + HO Ag+e Ag Co +eCo 1. 0.40 0.34 1.82 1.78 1,70 0.27 0.22 0.30 Ce+e Ce PO - 4H + SO + 2PASO, + 2H,0 MaO,+4H + 3Ma0, + ZH,0 2+ 2H + 10 →10," + HO Ma0,+ KH" + SeMa"+ 4H0 Au +3Au PhO, + 4H + 2e + 2H,0 016 168 2H + 2eB 0.00 -0.06 -Q13 160 131 P+2P Sa +2e Sa N4 2e- NI PASO, + P+ so, 10 -014 146 1.36 1.33 1,23 1.21 -035 Cr0, + 14H" + e2C" + 7HO 0,+4H + → 2H,0 MaOy + 4H +2e Ma + 2H,0 10, + GH + S4 + 3H,0 Bey+ 32Br VO," + 2H +e VO+ H0 AC+ 3 Au + 4a NO, +4H +3eNO + 21,0 -040 -041 -044 -G73 --ax 1.20 Z + 2- Za 2H,0 + 2e-H, + 201 Ma+ 2e Ma A+ Al H+ 2e2H Mg+2eMg La+ La Na" +e-Na C+ 2eCa 1.09 1.00 -LII 0.99 0.96 -166 -223 -237 0.954 0.91 2Hg + 2eHg" Ag +eAg H+2e"2Hg -2.37 -2.71 ON0 -276 -2.90 0.77 0.58 0, + 2H" + 2e -HO, MaO, Mo0, 6+ 2e" 21 -292 -3.05 K* +eK 0.56 0.4 0.52
Principles of Instrumental Analysis
7th Edition
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Chapter22: An Introduction To Electroanalytical Chemistry
Section: Chapter Questions
Problem 22.2QAP
Related questions
Question
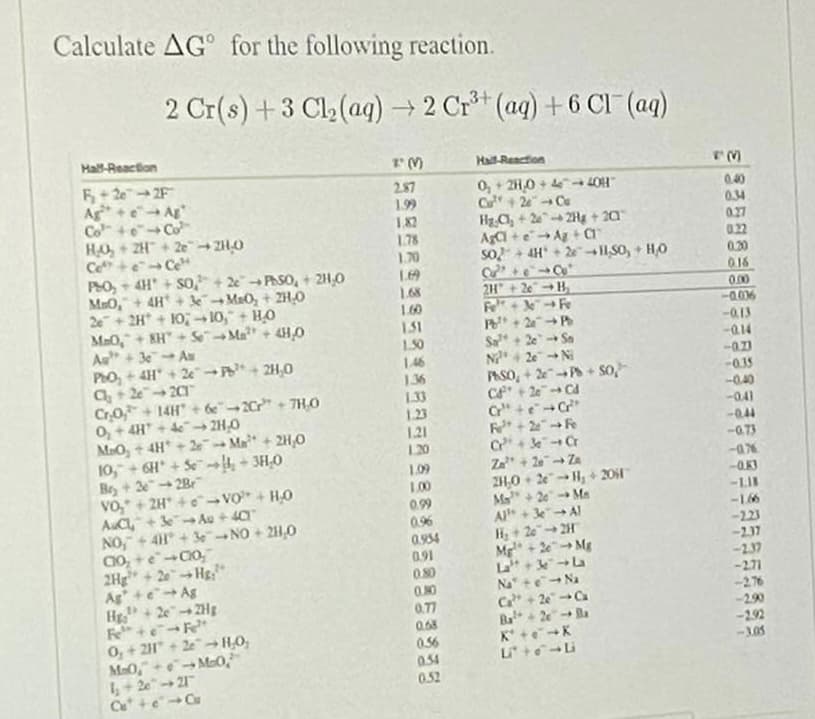
Transcribed Image Text:Calculate AG for the following reaction.
2 Cr(s) +3 Cl2(aq) 2 Cr*t (ag) + 6 CI (aq)
Hall-Reaction
Hall-Reaction
5+ 20 2F
Ag +e Ag
Co +eCo
HO, + 2H+ 2e 2H0
Ce+eCe
PO, - 4H + So,+ 2 PhSO, + 2H,0
MaO,+ 4H + 3MaO, + 2H,0
2+ 2H + 10; 10," + HO
Ma0,+ KH" + SeMa" + 4H,0
As +3 A
PhO, + 4H + 2e + 2H,0
C+ 2e 20
Cr0,+ 14H" + de2Cr" + 7H0
0, + 4H + → 2H,0
MaOy + 4H +2 Ma+ 2H,0
10, + 6H + Se 4 + 3H,0
Be+ 2-2B
vo," + 2H +e VO + H0
AuC+ 3 Au + 40
NO,+41 + 3eNO + 2H,0
2.87
0, + 2H0 + 4 4OH"
Cu+ 2 Cu
Hg C, + 22Hg + 20
Aa +e Ag +a
So+ 4H + 2I1S0, + HO
0.40
0.34
027
022
0.20
1.99
182
1.78
1.70
016
1,68
160
151
2H + 20-H
Fe + Fe
P+ 2-
0.00
-0.006
-0.13
150
Sa+ 2e
Sn
-Q14
N4 2e- N
PASO, + 2P+ So,
C + 2e" Cd
C" +eC
1.46
-035
-040
1.36
1.33
1.23
-041
1.21
1.20
-0.44
-G73
Za + 2 Za
2H,0 + 2e H, + 201
Ma"+ 2e Ma
1.09
100
-LI
0.99
0.96
0.954
0.91
0.80
A+3e
Al
-16
-223
-237
2Hg" + 2eHs"
Ag +eAg
H+2e2Hg
H + 2e 2H
Mg + 2eMg
La+3 La
Na" te-Na
Ca+ 2eCa
Ba+ 2Ba
K* +eK
-237
-271
-276
-290
-292
0, + 2H" + 2e-HO,
MaO,+ Ma0,
4+ 2e"21
C+eCu
0.77
0.68
0.56
0.54
052
-105
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning