Using values from the table of standard reduction potentials, calculate the cell potential (in V) of the following cells. 2+ (a) Co(s) | Co* (aq) || Ag" (aq) | Ag(s) 2+ 2+ (b) Cd(s)| Cd" (aq) || Cr^" (aq) | Cr(s) 2+ (c) Cd(s), CdS(s) |s´ (aq) || Sn™ (aq) | Sn(s) s²-
Using values from the table of standard reduction potentials, calculate the cell potential (in V) of the following cells. 2+ (a) Co(s) | Co* (aq) || Ag" (aq) | Ag(s) 2+ 2+ (b) Cd(s)| Cd" (aq) || Cr^" (aq) | Cr(s) 2+ (c) Cd(s), CdS(s) |s´ (aq) || Sn™ (aq) | Sn(s) s²-
Chemistry: The Molecular Science
5th Edition
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:John W. Moore, Conrad L. Stanitski
Chapter17: Electrochemistry And Its Applications
Section17.4: Voltaic Cells And Cell Potential
Problem 17.4PSP: Given this reaction, its standard potential, and the standard half-cell potential of 0.34 V for the...
Related questions
Question
999
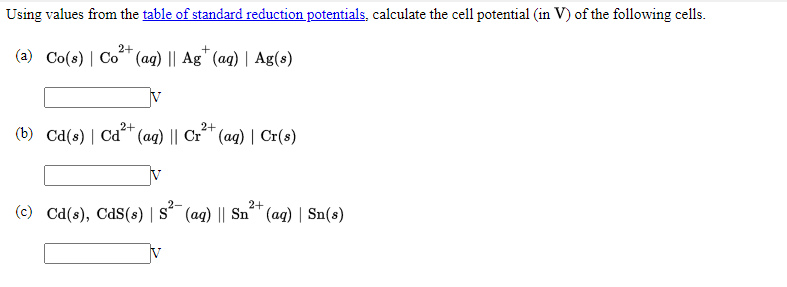
Transcribed Image Text:Using values from the table of standard reduction potentials, calculate the cell potential (in V) of the following cells.
2+
(a) Co(s) | Co (aq) || Ag" (aq) | Ag(s)
2+
2+
(b) Cd(s) | Cd (aq) || Cr´" (ag) | Cr(s)
V
s?-
2+
(c) Cd(s), CdS(s) | S´ (ag) || Sn" (ag) | Sn(s)
IV
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 4 steps with 3 images

Recommended textbooks for you

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning


Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning


Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning