Calculate AH and AUf for the formation of silane, SiH4(g), from its elements at 298 K, if 250 cm³ of the gaseous com- pound at T = 298 K and P = 0.658 atm is burned in a constant-volume gas calorimeter in an excess of oxygen and causes the evolution of 9.757 kJ of heat. The combus- tion reaction is SiH,(g) + 2 O2(g)→ SiO2(s, quartz) + 2 H,O(€) and the formation of silane from its elements is Si(s) + 2 H2(g) - · SİH4(g)
Calculate AH and AUf for the formation of silane, SiH4(g), from its elements at 298 K, if 250 cm³ of the gaseous com- pound at T = 298 K and P = 0.658 atm is burned in a constant-volume gas calorimeter in an excess of oxygen and causes the evolution of 9.757 kJ of heat. The combus- tion reaction is SiH,(g) + 2 O2(g)→ SiO2(s, quartz) + 2 H,O(€) and the formation of silane from its elements is Si(s) + 2 H2(g) - · SİH4(g)
Chemistry: Principles and Practice
3rd Edition
ISBN:9780534420123
Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Chapter5: Thermochemistry
Section: Chapter Questions
Problem 5.84QE
Related questions
Question
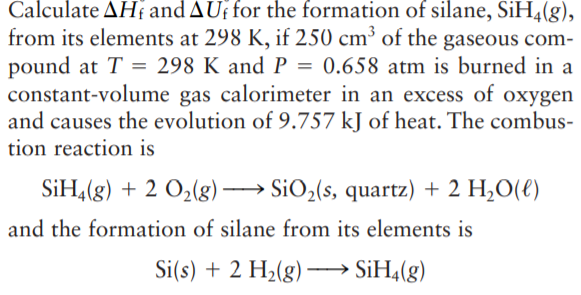
Transcribed Image Text:Calculate AH and AUf for the formation of silane, SiH4(g),
from its elements at 298 K, if 250 cm³ of the gaseous com-
pound at T = 298 K and P = 0.658 atm is burned in a
constant-volume gas calorimeter in an excess of oxygen
and causes the evolution of 9.757 kJ of heat. The combus-
tion reaction is
SiH,(g) + 2 O2(g)→ SiO2(s, quartz) + 2 H,O(€)
and the formation of silane from its elements is
Si(s) + 2 H2(g) -
· SİH4(g)
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 4 steps with 3 images

Recommended textbooks for you

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning