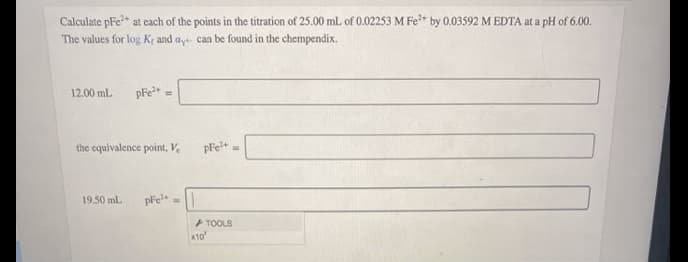
Calculate pFe at cach of the points in the titration of 25.00 mL of 0.02253 M Fe* by 0.03592 M EDTA at a pH of 6.00. The values for log Kr and ay can be found in the chempendix. 12.00 mL pFe the equivalence point, V, 19.50 ml. pre TOOLS x10
Q: 6. Calculate pl for the titration of 100.0 mL of 0.1023 M I´ with 0.1190 M AgNO3 for the addition of…
A: Reaction which will take place will be as follows: I- + AgNO3 -----> AgI + NO3- Once we start…
Q: 1. A 0.2700 g sample of impure Na,CO3 required 24.12 mL of 0.1684 M HCI and a back- titration with…
A: Solution -
Q: A student titrates a 50.00mL sample of water with 17.85mL of 0.0100M ethylenediaminetetraacetic acid…
A: Given: Volume of water sample = 50.00 mL Voluime of ethylenediaminetetraacetic acid (EDTA) =…
Q: 0,0585g Na2C2O4 10 mL 'to adjust KMnO4 solution prepared as 0,1M distilled water, 2 M H2SO4 was…
A:
Q: Conside the thration of f 0 mt cf n otco H (C N( weak tase, K64 05) with 0 100 C Cakulate the pt…
A: Since you have asked multiple questions, we will solve the first question for you. If you want any…
Q: Calculate pFe2+ at each of the points in the titration of 25.00 mL of 0.02165 M Fe2+ by 0.03614 M…
A:
Q: with 0.100 M NaOH. Calculate 1 A 50.00 mL aliquot of 0.0500 M acetic acid was titrated the pH after…
A: i) 0.00ml of NaOH CH3COOH <------> CH3COO-+ H+ Ka = [CH3COO ][H+ ]/[CH3COOH] = 1.78×10-5 at…
Q: Calculate pFe2+ at each of the points in the titration of 25.00 mL of 0.02233 M Fe2+ by 0.03516 M…
A:
Q: For the titration of 25.0mL 0.135MNH2NH2(aq)at 25°Cwith 0.135MHBr(aq), calculate the pH (a) at the…
A:
Q: 15.83 Consider the titration of 25.0 mL of 0.0200 M H,CO3 with 0.0250 M KOH. Calculate the pH after…
A: “Since you have posted a question with multiple sub-parts, we will solve first three subparts for…
Q: 0.0585g of Na2C2O4 10 mL of distilled water, 2 M H2SO4 were added to adjust the KMnO4 solution…
A: A titration is a technique that uses a known concentration of a solution to calculate the…
Q: 1. A 0.2700 g sample of impure NazCO3 required 24.12 mL of 0.1684 M HCI and a back- titration with…
A: 1. Na2CO3 + 2 HCl →2 NaCl + CO2 + H2O So, 2 mol HCl : 1 mol Na2CO3 Now, Moles of HCl used =…
Q: Calculate the equivalence point volumes for a titration of 25.00 mL of 0.060 M HCl and 01.0 M H3PO4…
A: HCl vs NaOH Volume of HCl ( Va ) =25.00 mL Molarity of HCl ( Ma ) = 0.060 M Molarity of NaOH ( Mb…
Q: A 100.0-mL aliquot of 0.100 M weak base B (pKb= 5.00) was titrated with 1.00 M HClO4 . Find the pH…
A: Since you have posted a question with multiple sub- parts, we will solve first three sub- parts for…
Q: Consider the titration of 30.0 mL of 0.20M nitrous acid by adding 0.0500 M aqueous ammonia to it.…
A:
Q: 3) Consider the titration of 40.0 mL of 0.100 M maleic acid (HzM) with 0.200 M NaOH. Maleic acid is…
A: Given: Volume of maleic acid i.e. H2M = 40.0 mL = 0.040 L (Since 1 L =…
Q: IKalculute the pH and plot the resulting titration curve for Hhe titration of 20ml, o.1l M ammonia…
A:
Q: Calculate the pH at the following points in a titration of 40. mL (0.040 L) of 0.145 M…
A: 4-chlorobenzoic acid is a weak acid. The chemical equation for the given question may be written as:…
Q: Consider the titration of 20.00 mL of 0.1145 M sodium azide (NaN3) with 0.1250 M HCl. The Ka of HN3…
A:
Q: Buffer dilution. 100 mL100 mL of a 0.1 mM0.1 mM buffer solution made from acetic acid and sodium…
A: Using the Henderson- Hasselbalch equation to find the ratio of [A-] to [HA] in the 100ml :…
Q: A buffer was made by mixing 0.1500 moles of HOBr with 0.1296 moles of NaOBr and diluting to exactly…
A: In the given buffer solution , HOBr is a weak acid and NaOBr is salt of conjugate base of HOBr.…
Q: Calculate pFe2+ at each of the points in the titration of 25.00 mL of 0.02042 M Fe2+ by 0.03684 M…
A:
Q: Consider the titration of 25ml of 0.0823M KI with 0.051M AgNO3, KspAgl =8.3x10-16 Calculate pAg*…
A: Given : Volume of KI = 25.00 mL Molarity of KI = 0.0823 M Molarity of AgNO3 = 0.051 M Ksp AgI = 8.3…
Q: A 30.0-mL volume of 0.50 M CH3COOH (Ka = 1.8 × 10-5) was titrated with 0.50 M NaOH. Calculate the pH…
A:
Q: pH Titration Curve for titrating 64.0 mL of Nitric Acid with 0.700 mol/L Aqueous Potassium Hydroxide…
A: From the pH vs volume of titrant we can figure out pH and Molarity of nitric acid.
Q: A 0.4071-g sample of CaCO3 (MM: 100.09 g/mole) is transferred to a 500-mL volumetric flask,…
A:
Q: Mercury thiocyanate - Hg(SCN)2 - is slightly soluble in water. Its Ksp is 2.8 x 10-20. In the…
A: Upon dilution, the number of moles in the solution remains constant whether the change in the…
Q: Calculate pFe+ at each of the points in the titration of 25.00 mL of 0.02019 M Fe by 0.03626 M EDTA…
A: Given: Volume of Fe2+ = 25.00 mL Concentration of Fe2+ = 0.02019 M Concentration of EDTA = 0.03626 M…
Q: Argentometric titration of a 0.7908-g sample containing chloride ion required 45.32 mL of 0.1046 M…
A:
Q: Calculate the pl after 50 mL of 0.1 M Nal is titrated with 20.0 mlL0.2 MAgNO, solution Caen E has…
A: The question is based on the concept of equilibrium. we have to calculate P value for iodide ion 8n…
Q: 3. This produces the observable physical change upon reaching the enc point of a titration indicator…
A: A point marking the completion of a reaction called end point
Q: 9.6 ml of a standard NaOH was consumed in the titration of 5.0 mL CH3COOH sample. Calculate for the…
A: Given: 1.0 mL of NaOH is equivalent to 0.1071 g of KHP. Volume of NaOH solution required = 9.6 mL.…
Q: Calculate the silver lon concentration in terms of pAg during the titration of 50.00 ml. of 0.05000…
A: Given in following question 50ml of 0.05000M Nacl with 0.1000M AgNO3 after the addition calculate…
Q: Suppose titration of 100.00 mL mixture of 0.0500 M in Br +0.08 M CI with 0.4000 M AgNO3. What is pAg…
A: Please find the below attachments.
Q: A 50.0-mL solution of 0.0319 M benzylamine was titrated with 0.0500 M HCl. Calculate the pH at the…
A: Henderson-Hasselbach equation. this equation shows the relationship between pH of a solution, the…
Q: 0.0585g of Na2C2O4 10 mL of distilled water, 2 M H2SO4 were added to adjust the KMnO4 solution…
A: A titration is a technique that uses a known concentration of a solution to calculate the…
Q: A titration curve was generated for the titration of 25.00 mL of 0.1000 MI- with 0.05000 M Ag*. The…
A: Since you have posted a question with multiple sub-parts, we will solve first three subparts for…
Q: 16.0mL of a 0.750M solution of H2CO3 are titrated with a 1.00M solution of KOH. At what volume of…
A:
Q: .Consider the titration of 25ml of 0.0823M KI with 0.051M AGNO3, Kspagi =8.3x10-16 Calculate pAg*…
A: The solubility product constant or the equilibrium constant is a measure of the extent to which the…
Q: Consider the following infermation about sulfurous acid, a diprotic id (11,SO) ELS03= HSO," + 1I*…
A: The titration curve given is, And the titration of Na2SO3 with HCl is given.
Q: A 20.0 mL solution of 0.200M weak acid HA is titrated with a 0.400M NaOH solution. (Ka of HA = 5.0 x…
A:
Q: Calculate the pl after 50 mL of 0.1 M Nal is titrated with 10.0 mL 0.2 M AGNO, solution? Given (AgI…
A: Given: Concentration of NaI = 0.1 M Concentration of AgNO3 = 0.1 M Volume of NaI = 50 mL Volume of…
Q: A 25.0 ml. sample of 0.250 M nitrous acidis titrated with a 0.250 M NAOH soluti on. What is the pH…
A: pH is defined as the negative logarithm of hydrogen ion concentration.
Q: A 100-mL solution of the ion Mg2+ at a concentration of 0.0500 M buffered to pH 9.00 was titrated…
A:
Q: A 15.00 ml aliquot of a 0.75 M solution of a weak acid (K, = 1.50E-04 at 25°C) is titrated with…
A:
Q: 4. A 100.0-mL solution consisting of 0.14 M NaC2H30z and 0.16 M HC2H302 was titrated with 24 mL of…
A: 4. Millimoles of KOH = M x V = 0.25 x 24 = 6 mmol Millimoles of HC2H3O2 = M x V = 0.16 x 100 = 16…
Q: If 21.6 mL of 0.107 M acid with a pKa of 5.16 is titrated with 0.105 M NaOH solution, what is the pH…
A: According to Henderson - Hasselbalch equation: pH=pKa+log[conjugate base][weak acid] At, half…
Q: Which of the following indicators will be the most appropriate to be used for the titration of 15.0…
A: Answer: In the acid-base titration, indicator is used to indicate that titration has been completed…
Q: 60ml of 0.1M HCN was titrated with 0.1M Na OH. Calculate the pHat a) initial b) 20ml c) 30ml d) 60ml…
A: Value of pH is used to show acidity or basicity of a solution. At room temperature, value of pH is…
Q: A 0.1 M solution of the weak acid HA was titrated with 0.1 M NaOH. The pH measured when Vbase=1/2…
A: The solution is given below -
Trending now
This is a popular solution!
Step by step
Solved in 5 steps

- The equilibrium constant for the conjugate acid-base pair HIn+H2OH3O++In is 8.00 10-5. From the additional information in the following table, (a) calculate the absorbance at 430 nmand 600 nm for the following indicator concentrations: 3.00 10-4M,2.00 10-4M, 1.00 10-4M, 0.500 10-4 M, and 0.250 10-4M. (b) plot absorbance as a function of indicator concentration.A Fajans titration of a 0.7908-g sample required 45.32 mL of 0.1046 M AgNO3 . Express the results of this analysis in terms of the percentage of BaCl2 * H2O. (Use a MW value in 4 decimal places)1. 1093-g sample of impure Na2CO3 was analyzed by residual precipitimetry. After adding 50.00 mL of 0.06911 M AgNO3, the sample was back-titrated with 0.05781 M KSCN, requiring 27.36 mL to reach the endpoint. The percentage Na2CO3 (MW = 106.0 g/mole) in the tested sample is ________ % ? Note: Express final answer using least number of significant figures. 2. The alkalinity of natural waters is usually controlled by OH- (MW = 17.01 g/mole), CO3-2 (MW = 60.01 g/mole), and HCO3- (MW = 61.01 g/mole), which may be present singularly or in combination. Titrating a 10.0-mL sample to a phenolphthalein endpoint requires 38.12 mL of a 0.5812 M solution of HCl, and an additional 18.67 mL of the same titrant to reach the methyl orange endpoint. The composition of the sample is _________% CO3-2 and ___________ % OH- Note: Express final answers using least number of significant figures.
- A sample is analyzed for chloride by the Volhard method. From the following data, calculate the percentage of chloride present:Weight of sample = 6.0000 g dissolved and diluted to 200 mLAliquot used = 25.00 mL AgNO3 added = 40.00ml of 0.1234MKSCN for back titration = 13.20ml of 0.0930MA 0.512 g sample of CaCO3 is dissolved in 12 M HCl and the mixture is diluted to 250 mL. A small amount of MgCl2 solution is added to a 25 mL aliquot of the solution., and the mixture is titrated with (ethylenediaminetetraacetic acid) EDTA to the Eriachrome Black T (with MgCl2 is used as indicator) end point. The mixed solution requires 28.55 mL of the EDTA solution to reach the end point. A similar amount of MgCl2 solution requires 2.60 mL to reach the endpoint. A 100-mL sample of hard water is titrated with 22.4 mL of the EDTA solution created above. The same amount of MgCl2 is added as previously and the total volume of EDTA solution required is 22.44 mL. a) Assume all of the Ca2+ in the water comes from CaCO3. How many moles of CaCO3 are in 1 L of water? How many grams of CaCO3 are in 1 L of water? c) If 1 ppm CaCO3 = 1 mg/liter, what is the water hardness in ppm CaCO3 ?A 0.512 g sample of CaCO3 is dissolved in 12 M HCl and the mixture is diluted to 250 mL. A small amount of MgCl2 solution is added to a 25 mL aliquot of the solution., and the mixture is titrated with (ethylenediaminetetraacetic acid) EDTA to the Eriachrome Black T (with MgCl2 is used as indicator) end point. The mixed solution requires 28.55 mL of the EDTA solution to reach the end point. A similar amount of MgCl2 solution requires 2.60 mL to reach the endpoint. A 100-mL sample of hard water is titrated with 22.4 mL of the EDTA solution created above. The same amount of MgCl2 is added as previously and the total volume of EDTA solution required is 22.44 mL. a) What volume of EDTA is used in titrating the Ca2+ in the hard water? b) How many moles of EDTA are there in that volume? c) How many moles of Ca2+ are in 100 mL of water?
- A 0.512 g sample of CaCO3 is dissolved in 12 M HCl and the mixture is diluted to 250 mL. A small amount of MgCl2 solution is added to a 25 mL aliquot of the solution., and the mixture is titrated with (ethylenediaminetetraacetic acid) EDTA to the Eriachrome Black T (with MgCl2 is used as indicator) end point. The mixed solution requires 28.55 mL of the EDTA solution to reach the end point. A similar amount of MgCl2 solution requires 2.60 mL to reach the endpoint. A 100-mL sample of hard water is titrated with 22.4 mL of the EDTA solution created above. The same amount of MgCl2 is added as previously and the total volume of EDTA solution required is 22.44 mL. How many moles of Ca2+ are in 100 mL of water?A 0.512 g sample of CaCO3 is dissolved in 12 M HCl and the mixture is diluted to 250 mL. A small amount of MgCl2 solution is added to a 25 mL aliquot of the solution., and the mixture is titrated with (ethylenediaminetetraacetic acid) EDTA to the Eriachrome Black T (with MgCl2 is used as indicator) end point. The mixed solution requires 28.55 mL of the EDTA solution to reach the end point. A similar amount of MgCl2 solution requires 2.60 mL to reach the endpoint. How many milliliters of EDTA are needed to titrate the Ca2+ ion in the sample? How many moles of EDTA are there in the volume calculated for the question above? What is the molarity of the EDTA solution?Solve the following problem: Calamine, which is used to relieve skin irritations, is a mixture of zinc and iron oxides. A 1.056 g sample of dry calamine was dissolved in acid and diluted to 250.0 mL. Potassium fluoride was added to a 10.00 mL aliquot of the diluted solution to mask the iron. After adjusting the pH, the Zn2+ consumed 38.37 mL of EDTA 0.01133 M. A second aliquot of 50.00 mL was buffered and titrated with 2.30 mL of a solution of ZnY2- 0.002647 M: Fe3+ + ZnY2- → FeY- + Zn2+ Calculate the percentages of ZnO and Fe2O3 in the sample.
- 2,5 ml volume has taken from an “hypothetic” solution which includes (3+) Sb and (3+) Fe and at the titration with 0.1004 N KMnO4, the wasted amount has found as 16,4 mL. The other 2,5 mL that has taken, has reduced with Zn after that, this 2,5 mL solution has titrates with the same KMnO4 solution solution and the wasted amount is 26,5mL. With these datas find the %concentrations of the ions at the solution.A 49.10 mL aliquot from a 0.500 L solution that contains 0.530 g of MnSO4 (MW=151.00 g/mol) required 41.6 mL of an EDTA solution to reach the end point in a titration. What mass, in milligrams, of CaCO3 ( MW=100.09 g/mol) will react with 1.53 mL of the EDTA solution?Chemistry A 20 mL solution containing both Ca2+ and Mg2+ cations is diluted in to 100 mL. When 10 mL of this solution is taken and titrated with 0.05 M EDTA at pH = 10 in the presence of Erio – T indicator, the consumption is found as 12 mL. A new 10 mL was taken from the same solution and (NH4)2C2O4 is added on it and then formed the precipitate is filtered. The filtered solution is titrated with the same EDTA solution and the consumption is found as 3 mL. So find the Ca2+ and Mg2+ amounts in the main sample solution in terms of mg/L.





