Calculate the amount of heat produced when 5.947 L of liquid glycerol (C,H,O,) is burned in excess oxygen gas according to the following thermochemical reaction: 2 C3H803 (1) + 7 02(g) → 6 CO2 (g) + 8 H20(g) AH = -3311 kJ The density of glycerol at 25°C is 1.261 g/mL. kJ
Calculate the amount of heat produced when 5.947 L of liquid glycerol (C,H,O,) is burned in excess oxygen gas according to the following thermochemical reaction: 2 C3H803 (1) + 7 02(g) → 6 CO2 (g) + 8 H20(g) AH = -3311 kJ The density of glycerol at 25°C is 1.261 g/mL. kJ
Chemistry: Principles and Practice
3rd Edition
ISBN:9780534420123
Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Chapter5: Thermochemistry
Section: Chapter Questions
Problem 5.84QE
Related questions
Question
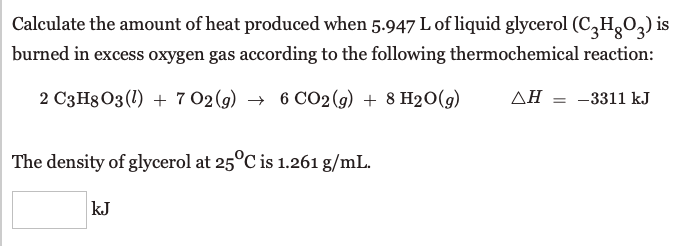
Transcribed Image Text:Calculate the amount of heat produced when 5.947 L of liquid glycerol (C,HgO,) is
burned in excess oxygen gas according to the following thermochemical reaction:
2 C3H8 03 (1) + 7 02 (g) → 6 CO2 (g) + 8 H20(g)
AH = -3311 kJ
The density of glycerol at 25°C is 1.261 g/mL.
kJ
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 5 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

World of Chemistry
Chemistry
ISBN:
9780618562763
Author:
Steven S. Zumdahl
Publisher:
Houghton Mifflin College Div

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

World of Chemistry
Chemistry
ISBN:
9780618562763
Author:
Steven S. Zumdahl
Publisher:
Houghton Mifflin College Div

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

EBK A SMALL SCALE APPROACH TO ORGANIC L
Chemistry
ISBN:
9781305446021
Author:
Lampman
Publisher:
CENGAGE LEARNING - CONSIGNMENT

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning