When methanol, CH,OH, is burned in the presence of oxygen gas, O,, a large amount of heat energy is released, as shown in the combustion reaction. CH, OH(g) + 0,(g)- CO,(g) + 2 H,0(1) AH = -764 kJ Based on the balanced thermochemical reaction, how much heat is produced when 33.1 g of methanol reacts with 51.3 g of oxygen? heat: kJ Which reactant is limiting? охygen methanol
When methanol, CH,OH, is burned in the presence of oxygen gas, O,, a large amount of heat energy is released, as shown in the combustion reaction. CH, OH(g) + 0,(g)- CO,(g) + 2 H,0(1) AH = -764 kJ Based on the balanced thermochemical reaction, how much heat is produced when 33.1 g of methanol reacts with 51.3 g of oxygen? heat: kJ Which reactant is limiting? охygen methanol
Chemistry: Principles and Reactions
8th Edition
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:William L. Masterton, Cecile N. Hurley
Chapter8: Thermochemistry
Section: Chapter Questions
Problem 65QAP: Natural gas companies in the United States use the therm as a unit of energy. One therm is 1105 BTU....
Related questions
Question
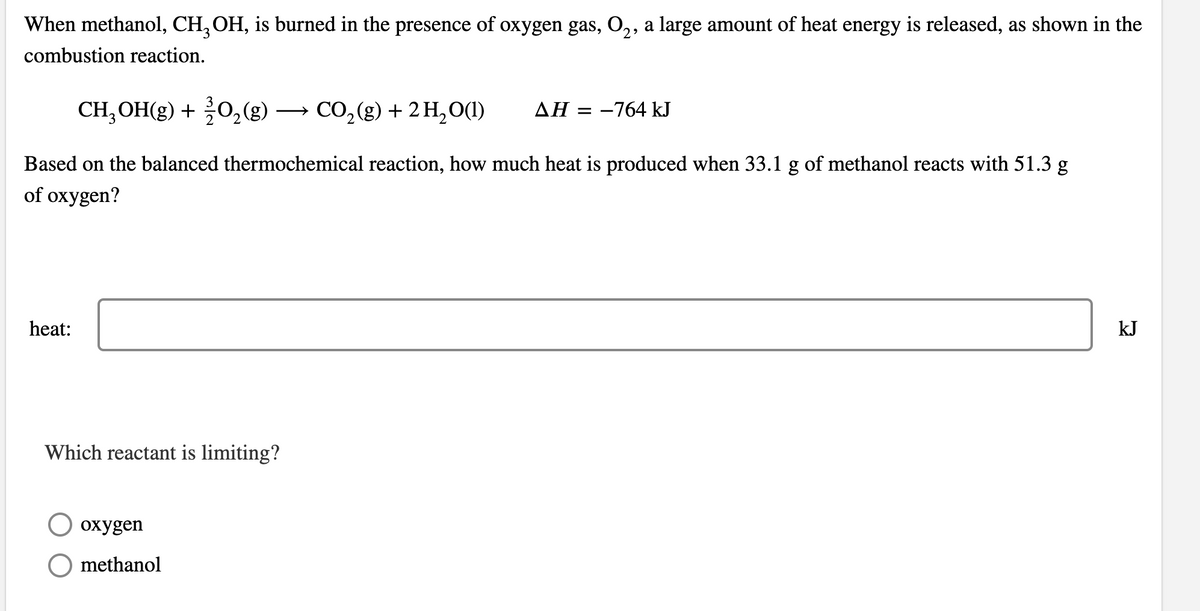
Transcribed Image Text:When methanol, CH, OH, is burned in the presence of oxygen gas, O,, a large amount of heat energy is released, as shown in the
combustion reaction.
CH;OH(g) + 0,(g) → CO,(g) + 2 H,0(1)
ΔΗ-764 kJ
Based on the balanced thermochemical reaction, how much heat is produced when 33.1 g of methanol reacts with 51.3 g
of oxygen?
heat:
kJ
Which reactant is limiting?
охудen
methanol
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Recommended textbooks for you

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax